Lab Anim Res.
2017 Jun;33(2):98-104. 10.5625/lar.2017.33.2.98.
Hyperglycemia decreases preoxiredoxin-2 expression in a middle cerebral artery occlusion model
- Affiliations
-
- 1Department of Anatomy, College of Veterinary Medicine, Research Institute of Life Science, Gyeongsang National University, 501 Jinjudaero, Jinju 660-701, Korea. pokoh@gnu.ac.kr
- KMID: 2407422
- DOI: http://doi.org/10.5625/lar.2017.33.2.98
Abstract
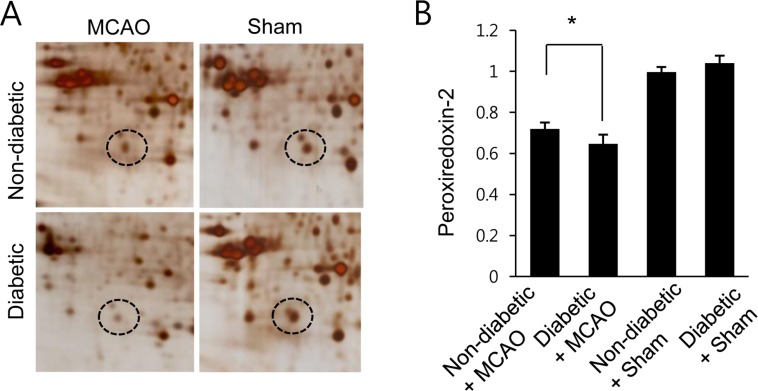
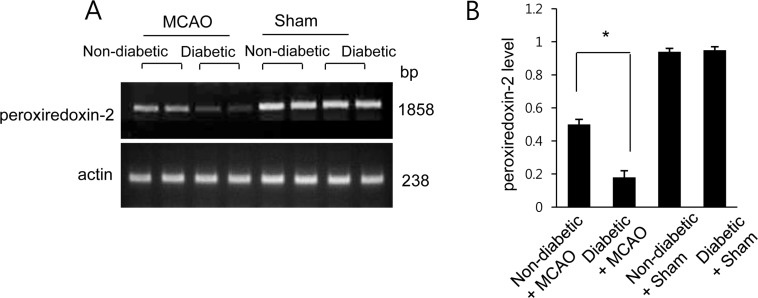
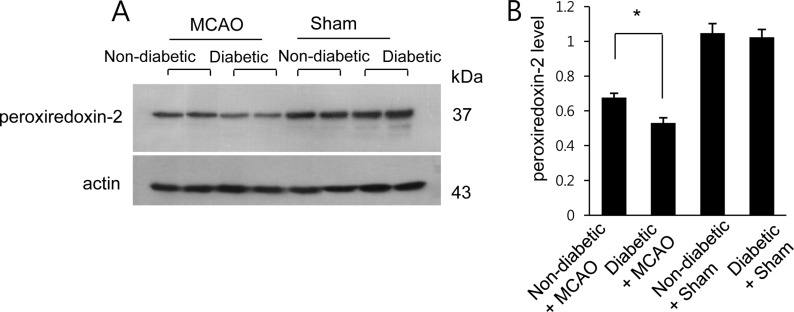
- Diabetes is a major risk factor for stroke and is also associated with worsened outcomes following a stroke. Peroxiredoxin-2 exerts potent neuroprotective effects against oxidative stress. In the present study, we identified altered peroxiredoxin-2 expression in an ischemic stroke model under hyperglycemic conditions. Adult male rats were administrated streptozotocin (40 mg/kg) via intraperitoneal injection to induce diabetes. Middle cerebral artery occlusion (MCAO) was induced surgically 4 weeks after streptozotocin treatment and cerebral cortex tissues were isolated 24 hours after MCAO. Peroxiredoxin-2 expression was evaluated in the cerebral cortex of MCAO-operated animals using a proteomics approach, and was found to be decreased. In addition, the reduction in peroxiredoxin-2 levels was more severe in cerebral ischemia with diabetes compared to animals without diabetes. Reverse-transcriptase PCR and Western blot analyses confirmed the significantly reduced peroxiredoxin-2 expression in MCAO-operated animals under hyperglycemic conditions. It is an accepted fact that peroxiredoxin-2 has antioxidative activity against ischemic injury. Thus, the findings of this study suggest that a more severe reduction in peroxiredoxin-2 under hyperglycemic conditions leads to worsened brain damage during cerebral ischemia with diabetes.
Keyword
MeSH Terms
-
Adult
Animals
Blotting, Western
Brain
Brain Ischemia
Cerebral Cortex
Humans
Hyperglycemia*
Infarction, Middle Cerebral Artery*
Injections, Intraperitoneal
Male
Middle Cerebral Artery*
Neuroprotective Agents
Oxidative Stress
Polymerase Chain Reaction
Proteomics
Rats
Risk Factors
Streptozocin
Stroke
Neuroprotective Agents
Streptozocin
Figure
Reference
-
1. Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001; 32(10):2426–2432. PMID: 11588337.2. Kamada H, Yu F, Nito C, Chan PH. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: relation to blood-brain barrier dysfunction. Stroke. 2007; 38(3):1044–1049. PMID: 17272778.3. Davidson JA, Parkin CG. Is hyperglycemia a causal factor in cardiovascular disease? Does proving this relationship really matter? Yes. Diabetes Care. 2009; 32(2):S331–S333. PMID: 19875575.4. Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011; 50(5):567–575. PMID: 21163346.
Article5. Won SJ, Tang XN, Suh SW, Yenari MA, Swanson RA. Hyperglycemia promotes tissue plasminogen activator-induced hemorrhage by Increasing superoxide production. Ann Neurol. 2011; 70(4):583–590. PMID: 22002675.
Article6. Sanderson TH, Reynolds CA, Kumar R, Przyklenk K, Huttemann M. Molecular mechanisms of ischemia-reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol Neurobiol. 2013; 47(1):9–23. PMID: 23011809.
Article7. Kalinina EV, Chernov NN, Saprin AN. Involvement of thio-, peroxi-, and glutaredoxins in cellular redox-dependent processes. Biochemistry (Mosc). 2008; 73(13):1493–1510. PMID: 19216714.
Article8. Chae HZ, Kang SW, Rhee SG. Isoforms of mammalian peroxiredoxin that reduce peroxides in presence of thioredoxin. Methods Enzymol. 1999; 300:219–226. PMID: 9919524.
Article9. Chae HZ, Oubrahim H, Park JW, Rhee SG, Chock PB. Protein glutathionylation in the regulation of peroxiredoxins: a family of thiol-specific peroxidases that function as antioxidants, molecular chaperones, and signal modulators. Antioxid Redox Signal. 2012; 16(6):506–523. PMID: 22114845.
Article10. Fisher AB. Peroxiredoxin 6: a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. Antioxid Redox Signal. 2011; 15(3):831–844. PMID: 20919932.11. Patenaude A, Murthy MR, Mirault ME. Emerging roles of thioredoxin cycle enzymes in the central nervous system. Cell Mol Life Sci. 2005; 62(10):1063–1680. PMID: 15761666.
Article12. Gan Y, Ji X, Hu X, Luo Y, Zhang L, Li P, Liu X, Yan F, Vosler P, Gao Y, Stetler RA, Chen J. Transgenic overexpression of peroxiredoxin-2 attenuates ischemic neuronal injury via suppression of a redox-sensitive pro-death signaling pathway. Antioxid Redox Signal. 2012; 17(5):719–732. PMID: 22356734.
Article13. Chen ML. Two-dimensional gel electrophoresis revealed antipsychotic drugs induced protein expression modulations in C6 glioma cells. Prog Neuropsychopharmacol Biol Psychiatry. 2013; 40:1–11. PMID: 22960606.
Article14. Hu X, Weng Z, Chu CT, Zhang L, Cao G, Gao Y, Signore A, Zhu J, Hastings T, Greenamyre JT, Chen J. Peroxiredoxin-2 protects against 6-hydroxydopamine-induced dopaminergic neurodegeneration via attenuation of the apoptosis signal-regulating kinase (ASK1) signaling cascade. J Neurosci. 2011; 31(1):247–261. PMID: 21209210.
Article15. Boulos S, Meloni BP, Arthur PG, Bojarski C, Knuckey NW. Peroxiredoxin 2 overexpression protects cortical neuronal cultures from ischemic and oxidative injury but not glutamate excitotoxicity, whereas Cu/Zn superoxide dismutase 1 overexpression protects only against oxidative injury. J Neurosci Res. 2007; 85(14):3089–3097. PMID: 17663478.
Article16. Koh PO. Proteomic analysis of focal cerebral ischemic injury in male rats. J Vet Med Sci. 2010; 72(2):181–185. PMID: 19942814.
Article17. Sung JH, Koh PO. Hyperglycemia aggravates decreases of PEA-15 and its two phosphorylated forms in cerebral ischemia. J Vet Med Sci. 2017; 79(3):654–660. PMID: 28216548.
Article18. Tancrede G, Rousseau-Migneron S, Nadeau A. Long-term changes in the diabetic state induced by different doses of streptozotocin in rats. Br J Exp Pathol. 1983; 64(2):117–123. PMID: 6221747.19. Ezquer M, Urzua CA, Montecino S, Leal K, Conget P, Ezquer F. Intravitreal administration of multipotent mesenchymal stromal cells triggers a cytoprotective microenvironment in the retina of diabetic mice. Stem Cell Res Ther. 2016; 7:42. PMID: 26983784.
Article20. Oh TW, Kang SY, Park YK. Histological analysis of five organs in streptozotocin-induced diabetic rats. Korean J Herbol. 2013; 28(6):39–45.
Article21. Wang H, Li H, Jiang X, Shi W, Shen Z, Li M. Hepcidin is directly regulated by insulin and plays an important role in iron overload in streptozotocin-induced diabetic rats. Diabetes. 2014; 63(5):1506–1518. PMID: 24379355.
Article22. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989; 20(1):84–91. PMID: 2643202.
Article23. Gim SA, Lee SR, Shah FA, Koh PO. Curcumin attenuates the middle cerebral artery occlusion-induced reduction in γ-enolase expression in an animal model. Lab Anim Res. 2015; 31(4):198–203. PMID: 26755923.
Article24. Rizk NN, Rafols J, Dunbar JC. Cerebral ischemia induced apoptosis and necrosis in normal and diabetic rats. Brain Res. 2005; 1053(1-2):1–9. PMID: 16038884.
Article25. Li ZG, Britton M, Sima AA, Dunbar JC. Diabetes enhances apoptosis induced by cerebral ischemia. Life Sci. 2004; 76(3):249–262. PMID: 15531378.
Article26. Haskins K, Bradley B, Powers K, Fadok V, Flores S, Ling X, Pugazhenthi S, Reusch J, Kench J. Oxidative stress in type 1 diabetes. Ann N Y Acad Sci. 2003; 1005:43–54. PMID: 14679039.
Article27. Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, Yagihashi S. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia. 2002; 45(1):85–96. PMID: 11845227.
Article28. Zhao F, Wang Q. The protective effect of peroxiredoxin II on oxidative stress induced apoptosis in pancreatic β-cells. Cell Biosci. 2012; 2(1):22. PMID: 22709359.
Article29. Nukatsuka M, Sakurai H, Yoshimura Y, Nishida M, Kawada J. Enhancement by streptozotocin of O2- radical generation by the xanthine oxidase system of pancreatic beta-cells. FEBS Lett. 1988; 239(2):295–298. PMID: 2846360.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intracranial Cerebrovascular Revascularization(Extracranial-Intracranial Arterial Bypass, EIAB)
- The Time Evolution of Cerebral Apoptosis in the Permanent Middle Cerebral Artery Occlusion Model in Rats
- Hyperglycemia aggravates decrease in alpha-synuclein expression in a middle cerebral artery occlusion model
- Traumatic Occlusion of the Middle Cerebral Artery
- Ischemic brain injury decreases dynamin-like protein 1 expression in a middle cerebral artery occlusion animal model and glutamate-exposed HT22 cells