J Neurocrit Care.
2017 Dec;10(2):53-59. 10.18700/jnc.170035.
Pharmacologic Characteristics of Corticosteroids
- Affiliations
-
- 1Department of Pharmacy, Memorial Hermann Texas Medical Center, Houston, TX, USA.
- 2Department of Neurosurgery and Neurology, The University of Texas Medical School at Houston, Houston, TX, USA. Huimahn.A.Choi@uth.tmc.edu
- KMID: 2405758
- DOI: http://doi.org/10.18700/jnc.170035
Abstract
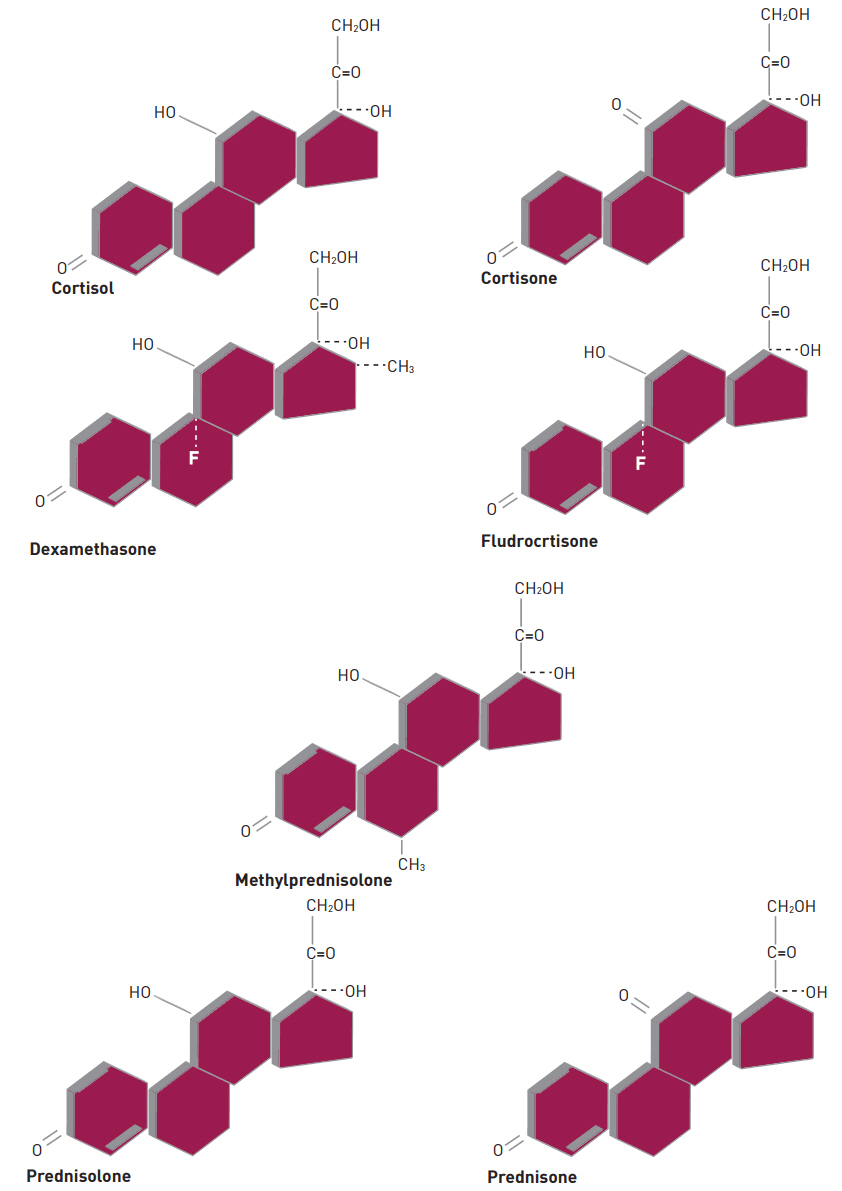
- Corticosteroids (CSs) are used frequently in the neurocritical care unit mainly for their anti-inflammatory and immunosuppressive effects. Despite their broad use, limited evidence exists for their efficacy in diseases confronted in the neurocritical care setting. There are considerable safety concerns associated with administering these drugs and should be limited to specific conditions in which their benefits outweigh the risks. The application of CSs in neurologic diseases, range from traumatic head and spinal cord injuries to central nervous system infections. Based on animal studies, it is speculated that the benefit of CSs therapy in brain and spinal cord, include neuroprotection from free radicals, specifically when given at a higher supraphysiologic doses. Regardless of these advantages and promising results in animal studies, clinical trials have failed to show a significant benefit of CSs administration on neurologic outcomes or mortality in patients with head and acute spinal injuries. This article reviews various chemical structures between natural and synthetic steroids, discuss its pharmacokinetic and pharmacodynamic profiles, and describe their use in clinical practice.
MeSH Terms
Figure
Cited by 1 articles
-
The impact of single dose intravenous dexamethasone as an adjunctive therapy for primary treatment on concentrations of inflammatory biomarkers in children with Kawasaki disease
Jung Eun Kwon
Pediatr Emerg Med J. 2022;9(1):23-28. doi: 10.22470/pemj.2022.00423.
Reference
-
1. Schimmer BP, Funder JW. ACTH, Adrenal Steroids, and Pharmacology of the Adrenal Cortex. In : Brunton LL, Chabner BA, Knollmann BC, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York: McGraw-Hill;2011. p. 1209–35.2. Hench PS, Kendall EC, Slocumb CH, Polley HF. The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocorticosterone; compound E) and of pituitary adrenocorticotropic hormone on rheumatoid arthritis. Proc Staff Meet Mayo Clin. 1949; 24:181–97.3. Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005; 353:1711–23.4. Vischer TL, Sinniger M, Ott H, Gerster JC. A randomized, double-blind trial comparing a pulse of 1000 with 250 mg methylprednisolone in rheumatoid arthritis. Clin Rheumatol. 1986; 5:325–6.
Article5. Garber EK, Fan PT, Bluestone R. Realistic guidelines of corticosteroid therapy in rheumatic disease. Semin Arthritis Rheum. 1981; 11:231–56.
Article6. Laan RF, van Riel PL, van de Putte LB, van Erning LJ, van’t Hof MA, Lemmens JA. Low-dose prednisone induces rapid reversible axial bone loss in patients with rheumatoid arthritis. A randomized, controlled study. Ann Intern Med. 1993; 119:963–8.7. Migeon CJ, Lawrence B, Bertrand J, Holman GH. In vivo distribution of some 17-Hydroxycorticosterioids between the plasma and red blood cells of man. J Clin Endocrinol Metab. 1959; 19:1411–9.8. Pugeat MM, Dunn JF, Nisula BC. Transport of steroid hormones: interaction of 70 drugs with testosterone-binding globulin and corticosteroid-binding globulin in human plasma. J Clin Endocrinol Metab. 1981; 53:69–75.
Article9. Ballard PL. Delivery and transport of glucocorticoids to target cells. Monogr Endocrinol. 1979; 12:25–48.
Article10. Diederich S, Eigendorff E, Burkhardt P, Quinkler M, Bumke- Vogt C, Rochel M, et al. 11beta-hydroxysteroid dehydrogenase types 1 and 2: an important pharmacokinetic determinant for the activity of synthetic mineralo- and glucocorticoids. J Clin Endocrinol Metab. 2002; 87:5695–701.11. Koetz KR, van Rossum EFC, Ventz M, Diederich S, Quinkler M. BclI polymorphism of the glucocorticoid receptor gene is associated with increased bone resorption in patients on glucocorticoid replacement therapy. Clin Endocrinol (Oxf). 2013; 78:831–7.
Article12. Giordano R, Marzotti S, Berardelli R, Karamouzis I, Brozzetti A, D’Angelo V, et al. BClI polymorphism of the glucocorticoid receptor gene is associated with increased obesity, impaired glucose metabolism and dyslipidaemia in patients with Addison’s disease. Clin Endocrinol (Oxf). 2012; 77:863–70.
Article13. Meikle AW, Weed JA, Tyler FH. Kinetics and interconversion of prednisolone and prednisone studied with new radioimmunoassays. J Clin Endocrinol Metab. 1975; 41:717–21.
Article14. Meikle AW, Tyler FH. Potency and duration of action of glucocorticoids. Effects of hydrocortisone, prednisone and dexamethasone on human pituitary-adrenal function. Am J Med. 1977; 63:200–7.15. Liddle GW. Studies of structure-function relationships of steroids. II. The 6 alpha-methylcorticosteroids. Metabolism. 1958; 7(4 Pt 2):405–15.16. Dulin WE. Anti-inflammatory activity of delta1-9alpha-fluorohydrocortisone acetate. Proc Soc Exp Biol Med. 1955; 90:115–7.17. Soffer LJ, Orr RH. Symposium: newer hydrocortisone analogs. Metabolism. 1958; 7(4 Pt 2):383–6.18. Dulin WE, Barnes LE, Glenn EM, Lyster SC, Collins EJ. Biologic activities of some C21 steroids and some 6 alpha-methyl C21 steroids. Metabolism. 1958; 7(4 Pt 2):398–404.19. Fauci AS. Mechanisms of the Immunosuppressive and antiinflammatory effects of glucorcorticosteroids. J Immunopharmacol. 1978-1979; 1:1–25.
Article20. Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011; 335:2–13.
Article21. Yokoyama H, Takabatake T, Takaeda M, Wada T, Naito T, Ikeda K, et al. Up-regulated MHC-class II expression and γ-IFN and soluble IL-2R in lupus nephritis. Kidney Int. 1992; 42:755–63.
Article22. Kehlet H, Binder C, Blichert-Toft M. Glucocorticoid maintenance therapy following adrenalectomy: assessment of dosage and preparation. Clin Endocrinol (Oxf). 1976; 5:37–41.
Article23. Roberts I, Yates D, Sandercock P, Farrell B, Wasserberg J, Lomas G, et al. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial. Lancet. 2004; 364:1321–8.24. Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GW, Bell MJ, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017; 80:6–15.
Article25. Olby N. Current concepts in the management of acute spinal cord injury. J Vet Intern Med. 1999; 13:399–407.
Article26. Buttgereit F, Wehling M, Burmester GR. A new hypothesis of modular glucocorticoid actions: Steroid treatment of rheumatic diseases revisited. Arthritis Rheum. 1998; 41:761–7.
Article27. Bracken MB, Collins WF, Freeman DF, Shepard MJ, Wagner FW, Silten RM, et al. Efficacy of methylprednisolone in acute spinal cord injury. JAMA. 1984; 251:45–52.
Article28. Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinalcord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990; 322:1405–11.29. Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997; 277:1597–604.
Article30. de Gans J, van de Beek D; European Dexamethasone in Adulthood Bacterial Meningitis Study Investigators. Dexamethasone in adults with bacterial meningitis. N Engl J Med. 2002; 347:1549–56.
Article31. van de Beek D, Farrar JJ, de Gans J, Mai NT, Molyneux EM, Peltola H, et al. Adjunctive dexamethasone in bacterial meningitis: a meta-analysis of individual patient data. Lancet Neurol. 2010; 9:254–63.
Article32. Wong EC, Brown G. Does pulse dosing of methylprednisolone have an acute effect on serum creatinine concentrations? Can J Hosp Pharm. 2014; 67:458–9.
Article33. Isenberg DA, Morrow WJ, Snaith ML. Methyl prednisolone pulse therapy in the treatment of systemic lupus erythematosus. Ann Rheum Dis. 1982; 41:347–51.
Article34. National Multiple Sclerosis Society. Recommendations Regarding Corticosteroids in the Management of Multiple Sclerosis Management of Relapses [online]. Available at: http://www.nationalmssociety.org/NationalMSSociety/media/MSNationalFiles/Brochures/ExpOp_Steroids.pdf. Accessed 2014.35. Buchman AL. Side effects of corticosteroid therapy. J Clin Gastroenterol. 2001; 33:289–94.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pharmacologic Treatment of Atopic Dermatitis
- Guidelines for Clinical Management of Premenstrual Syndrome
- Two Cases of Stevens-Johnson Syndrome Caused by Systemic Corticosteroids
- Inhaled Corticosteroids in Asthma
- Pharmacologic Therapies for Preventing Chronic Obstructive Pulmonary Disease Exacerbations: A Comprehensive Review