J Korean Med Assoc.
2007 Oct;50(10):894-902. 10.5124/jkma.2007.50.10.894.
Inhaled Corticosteroids in Asthma
- Affiliations
-
- 1Department of Internal Medicine, Kyungpook University School of Medicine, Korea. jomlee@knu.ac.kr
- KMID: 2065001
- DOI: http://doi.org/10.5124/jkma.2007.50.10.894
Abstract
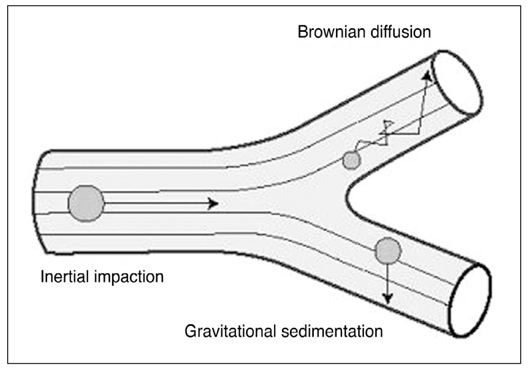
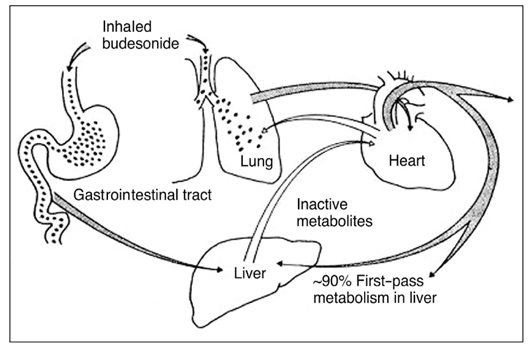
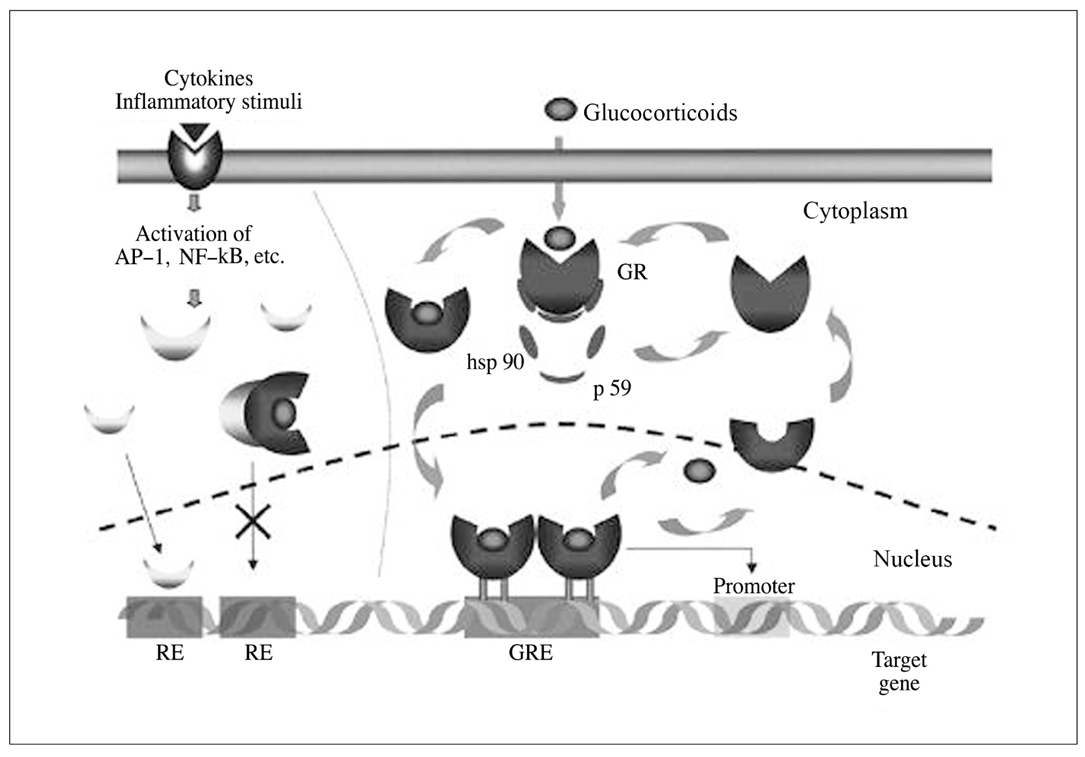
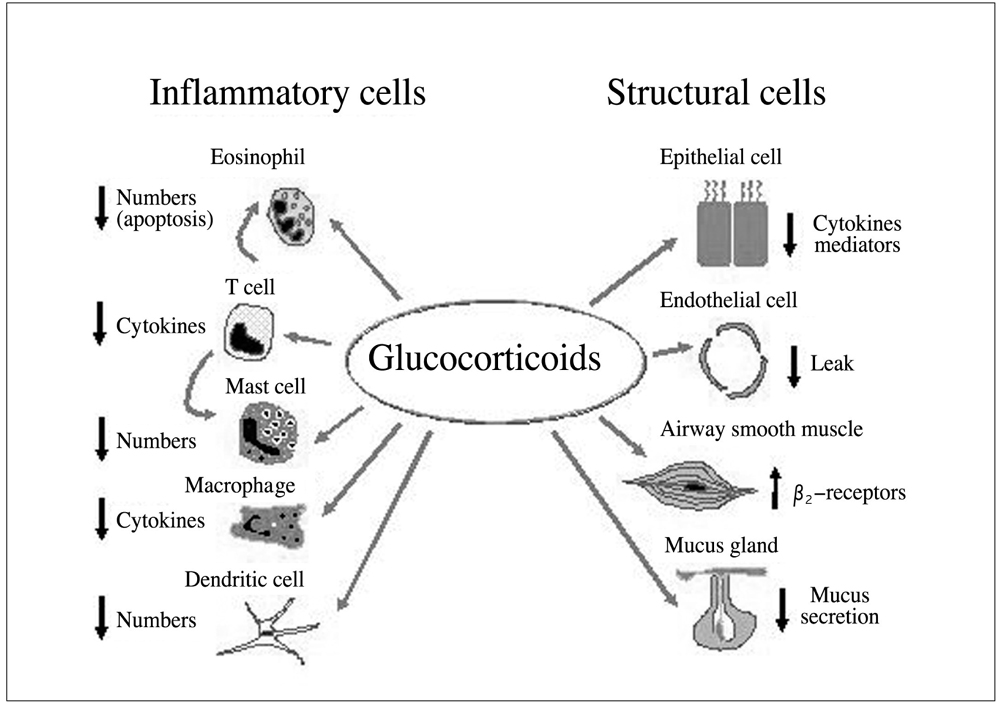
- Asthma is characterized by airway inflammation, hyperresponsiveness, and remodeling, which are responsible for the variable airway obstruction and respiratory symptoms. Although a number of anti-asthma drugs have been developed, none has succeeded in managing symptoms and underlying airway inflammation as effectively as corticosteroids. However, systemic effects of the drugs were a major obstacle to use them in asthma therapy. A significant breakthrough in asthma therapy occurred when corticosteroids became available as inhaled preparations, reducing the potential for systemic effects while achieving excellent therapeutic efficacy. The first inhaled corticosteroid (ICS) having such characteristics was beclomethasone-17alpha, 21-dipropionate, which was introduced in the early 1970s. With growing information on their efficacy and the recognition of chronic inflammation as a fundamental component of asthma, ICSs have become the cornerstone of asthma therapy since the early 1990s. The potent anti-inflammatory effect of ICSs seems to be resulted mainly from the reduction of the number and activation of inflammatory cells in the bronchial mucosa and through their inhibitory effects on the synthesis of various mediators and cytokines. In addition, the airway selectivity is thought to come from a high hepatic first-pass inactivation rate of the drugs. A number of ICSs are currently on the market, and some drugs are available as the combination of an ICS and a long-acting beta2 agonist in a single inhaler. The present paper comprises a short background behind the development of ICSs, their pharmacologic and clinical profiles, and the current positions in asthma treatment.
Keyword
MeSH Terms
Figure
Reference
-
1. Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000. 161:1720–1745.2. Pauwels RA, Pedersen S, Busse WW, Tan WC, Chen YZ, Ohlsson SV, Ullman A, Lamm CJ, O'Byrne PM. START Investigators Group. Early intervention with budesonide in mild persistent asthma: a randomised, double-blind trial. Lancet. 2003. 361:1071–1076.
Article3. Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy: A systematic review and meta-analysis. Arch Intern Med. 1999. 159:941–955.
Article4. Levi G. Targeted drug delivery-some pharmacokinetic considerations. Pharm Res. 1987. 4:3–4.5. Robertson DB, Maibach HI. Schleimer RP, Claman HN, Oronsky AL, editors. Topical glucocorticoids. Anti-inflammatory Steroid Action. Basic and Clinical Aspects. 1989. San Diego: Academic Press;494–524.6. Shigel SC, Heimlich EM, Richards W, Kelly VC. Adrenal function in allergy I. Effect of dexamethasone aerosols in asthmatic children. J Pediatr. 1964. 33:245.7. Brown HM, Storey G, George WHS. Beclomethasone dipropionate: a new steroid aerosol for the treatment of allergic asthma. Br Med J. 1972. 1:585–590.
Article8. Martin LE, Tanner RJN, Clark TJH, Cochrane GM. Absorption and metabolism of orally administered beclomethasone dipropionate. Clin Pharmacol Ther. 1974. 15:267.
Article9. Newman SP. A comparison of lung deposition patterns between different asthma inhalers. J Aerosol Med. 1995. 8:S3. 21–26.
Article10. Brattsand R. Schleimer RP, O'Byurne PM, Szefler SJ, Brattsand R, editors. Drug Development of Inhaled Steroids. A Pharmacologist's View Based on Experiences from the Budesonide Project. Inhaled Steroids in Asthma. Optimizing Effects in the Airways. 2002. New York: Marcel Dekker;3–32.11. Tunek A, Sjodin K, Hallstrom G. Reversible formation of fatty acid esters of budesonide, an antiasthma glucocorticoid, in human lung and liver microsomes. Drug Metab Dispos. 1997. 25:1311–1317.12. Ventresca G, Mackie A, Moss J, McDowall J, Bye A. Absorption of oral fluticasone propionate in healthy subjects. Am J Respir Crit Care Med. 1994. 149:A214.13. Rohagagi S, Rhodes GR, Chaikin P. Absolute oral versus inhaled bioavailability: significance for inhaled drugs with special reference to inhaled glucocorticoids. J Clin Pharmacol. 1999. 39:661–663.
Article14. Adcock IM, Ito K. Molecular mechanisms of corticosteroid actions. Monaldi Arch Chest Dis. 2000. 55:256–266.15. Denburg JA, Leber BF. Townley RG, Agrawal DK, editors. Inflammatory Cell Differentiation and Maturation in Allergic Reactions. Immunopharmacology of Allergic Diseases. 1996. New York: Marcel Dekker;139–157.16. Pelaia G, Vatrella A, Cuda G, Maselli R, Marsico SA. Molecular mechanisms of corticosteroid actions in chronic inflammatory airway diseases. Life Sci. 2003. 72:1549–1561.
Article17. Juniper EF, Kline PA, Vanzieleghem MA, Ramsdale EH, O'Byrne PM, Hargreave FE. Effect of long-term treatment with an inhaled corticosteroid (budesonide) on airway hyper-responsiveness and clinical asthma in nonsteroid-dependent asthmatics. Am Rev Respir Dis. 1990. 142:832–836.
Article18. Jeffery PK, Godfrey RW, Adelroth E, Nelson F, Rogers A, Johansson SA. Effects of treatment on airway inflammation and thickening of basement membrane reticular collagen in asthma. A quantitative light and electron microscopic study. Am Rev Respir Dis. 1992. 145:890–899.
Article19. Pauwels RA, Löfdahl CG, Postma DS, Tattersfield AE, O'Byrne P, Barnes PJ, Ullman A. Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. N Engl J Med. 1997. 337:1405–1411.
Article20. Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. 2000. 343:332–336.
Article21. Powell H, Gibson PG. Inhaled corticosteroid doses in asthma: an evidence-based approach. Med J Aust. 2003. 178:223–225.
Article22. Price DB, Hernandez D, Magyar P, Fiterman J, Beeh KM, James IG, Konstantopoulos S, Rojas R, van Noord JA, Pons M, Gilles L, Leff JA. Clinical Outcomes with Montelukast as a Partner Agent to Corticosteroid Therapy (COMPACT) International Study Group. Randomised controlled trial of montelukast plus inhaled budesonide versus double dose inhaled budesonide in adult patients with asthma. Thorax. 2003. 58:211–216.
Article23. Evans DJ, Taylor DA, Zetterstrom O, Chung KF, O'Connor BJ, Barnes PJ. A comparison of low-dose inhaled budesonide plus theophylline and high-dose inhaled budesonide for moderate asthma. N Engl J Med. 1997. 337:1412–1418.
Article24. Woolcock A, Lundback B, Ringdal N, Jacques LA. Comparison of addition of salmeterol to inhaled steroids with doubling of the dose of inhaled steroids. Am J Respir Crit Care Med. 1996. 153:1481–1488.
Article25. Shrewsbury S, Pyke S, Britton M. Meta-analysis of increased dose of inhaled steroid or addition of salmeterol in symptomatic asthma (MIASMA). BMJ. 2000. 320:1368–1373.
Article26. Boulet LP, Drollmann A, Magyar P, Timar M, Knight A, Engelstatter R, Fabbri L. Comparative efficacy of once-daily ciclesonide and budesonide in the treatment of persistent asthma. Respir Med. 2006. 100:785–794.
Article27. Toogood JH, Baskerville J, Jennings B, Lefcoe NM, Johansson SA. Influence of dosing frequency and schedule on the response of chronic asthmatics to the aerosol steroid budesonide. J Allergy Clin Immunol. 1982. 70:288–298.
Article28. Rabe KF, Pizzichini E, Ställberg B, Romero S, Balanzat AM, Atienza T, Lier PA, Jorup C. Budesonide/formoterol in a single inhaler for maintenance and relief in mild-to-moderate asthma: a randomized, double-blind trial. Chest. 2006. 129:246–256.
Article29. Grahnen A, Brundin RM, Ling-Andersson A, Lonnebro A, Eckernas SA. The systemic potency of fluticasone propionate from the Diskhaler vs budesonide from Turbuhaler. Am J Respir Crit Care Med. 1996. 153:A338.30. Bahceciler NN, Nuhoglu Y, Nursoy MA, Kodalli N, Barlan IB, Basaran MM. Inhaled corticosteroid therapy is safe in tuberculin-positive asthmatic children. Pediatr Infect Dis J. 2000. 19:215–218.
Article31. Lipworth BJ, Kaliner MA, LaForce CF, Baker JW, Kaiser HB, Amin D, Kundu S, Williams JE, Engelstaetter R, Banerji DD. Effect of ciclesonide and fluticasone on hypothalamicpituitary-adrenal axis function in adults with mild to moderate persistent asthma. Ann Allergy Asthma Immunol. 2005. 94:465–472.
Article32. National Heart Lung and Blood Institute. Global Initiative for Asthma: Global strategy for asthma management and prevention, Rev. 2006. 2006. Bethsda, MD: U.S Dept. of Health and Human Services, Public Health Services.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhaled Corticosteroids Is Not Associated with the Risk of Pneumonia in Asthma
- Treatment of mild asthma: Is it necessary to keep regular inhaled corticosteroids?
- An experimental asthma exacerbation following discontinuation of inhaled corticosteroids treatment in patients with controlled asthma
- Progress in the management of childhood asthma
- Add-on Therapy for Symptomatic Asthma despite Long-Acting Beta-Agonists/Inhaled Corticosteroid