Clin Orthop Surg.
2018 Mar;10(1):99-110. 10.4055/cios.2018.10.1.99.
Enhanced Tendon-to-Bone Healing of Chronic Rotator Cuff Tears by Bone Marrow Aspirate Concentrate in a Rabbit Model
- Affiliations
-
- 1Department of Orthopaedic Surgery, Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea. happynoh@gmail.com
- 2Department of Orthopaedic Surgery, The Second Hospital of Jilin University, Changchun, China.
- 3Department of Orthopedic Surgery, The Armed Forces Daejeon Hospital, Daejeon, Korea.
- 4Gachon Medical Research Institute, Gil Medical Center, Gachon University, Incheon, Korea.
- KMID: 2405489
- DOI: http://doi.org/10.4055/cios.2018.10.1.99
Abstract
- BACKGROUND
To evaluate the influence of bone marrow aspirate concentrate (BMAC) on tendon-to-bone healing in a rabbit rotator cuff model and to characterize the composition of growth factors in BMAC.
METHODS
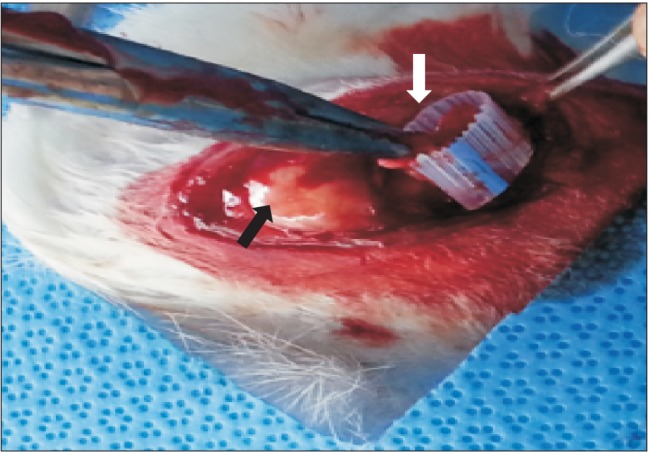
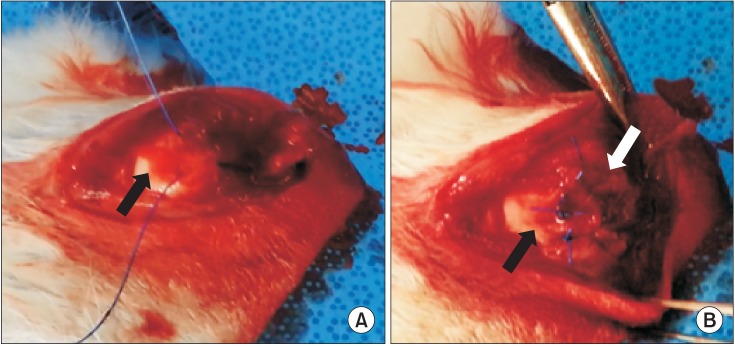
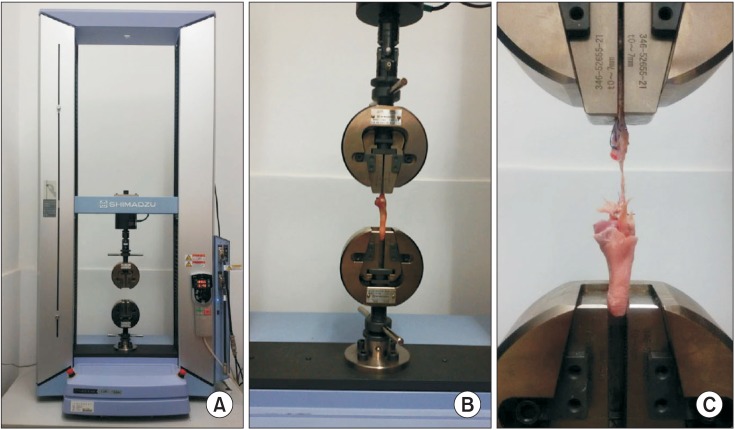
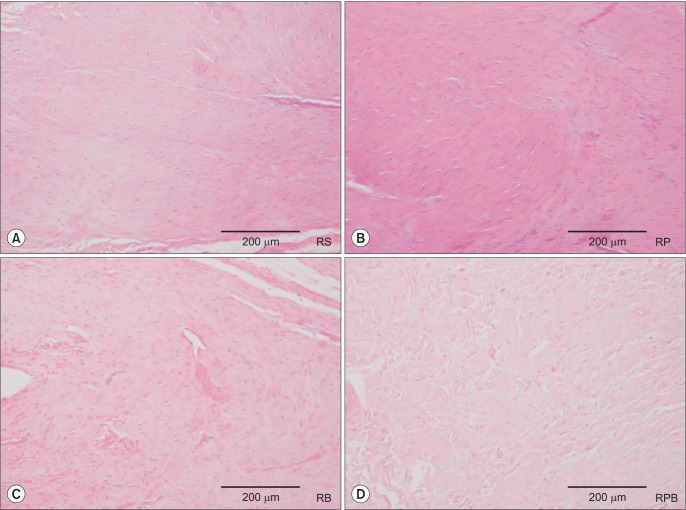
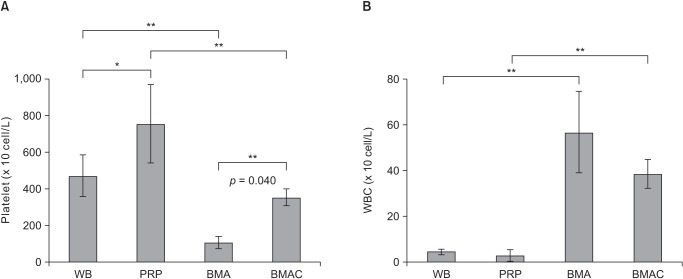
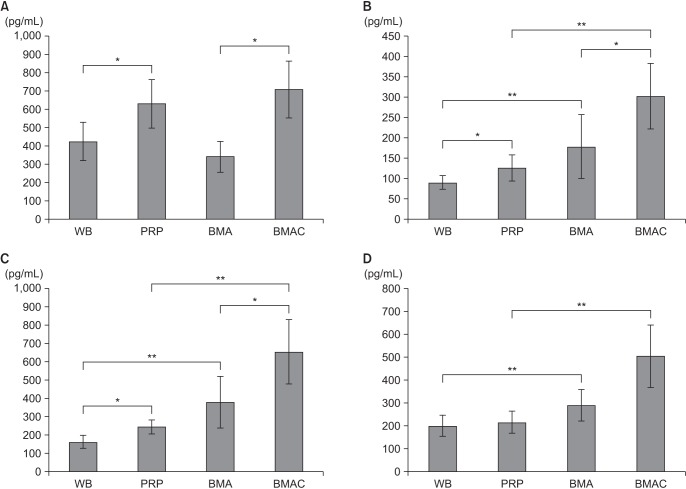
In this in vivo study, 40 rabbits were allocated into five groups: control (C), repair + saline (RS), repair + platelet-rich plasma (PRP; RP), repair + BMAC (RB) and repair + PRP + BMAC (RPB). A tear model was created by supraspinatus tendon transection at the footprint. Six weeks after transection, the torn tendon was repaired along with BMAC or PRP administration. Six weeks after repair, shoulder samples were harvested for biomechanical and histological testing. Ten rabbits were used for processing PRP and BMAC, followed by analysis of blood cell composition and the levels of growth factors in vitro.
RESULTS
The ultimate load-to-failure was significantly higher in RPB group compared to RS group (p = 0.025). BMAC-treated groups showed higher values of biomechanical properties than RS group. The histology of BMAC-treated samples showed better collagen fiber continuity and orientation than RS group. BMAC contained significantly higher levels of the several growth factors than PRP.
CONCLUSIONS
Locally administered BMAC enhanced tendon-to-bone healing and has potential for clinical applications.
Keyword
MeSH Terms
Figure
Reference
-
1. Seida JC, LeBlanc C, Schouten JR, et al. Systematic review: nonoperative and operative treatments for rotator cuff tears. Ann Intern Med. 2010; 153(4):246–255. PMID: 20621893.
Article2. Harryman DT 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA 3rd. Repairs of the rotator cuff: correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991; 73(7):982–989. PMID: 1874784.
Article3. Lorbach O, Pape D, Raber F, Kohn D, Kieb M. Arthroscopic rotator cuff repair using a single-row of triple-loaded suture anchors with a modified suture configuration. Arch Orthop Trauma Surg. 2011; 131(8):1073–1076. PMID: 21373919.
Article4. Livermore RW, Chong AC, Prohaska DJ, Cooke FW, Jones TL. Knot security, loop security, and elongation of braided polyblend sutures used for arthroscopic knots. Am J Orthop (Belle Mead NJ). 2010; 39(12):569–576. PMID: 21720573.5. Gerber C, Schneeberger AG, Perren SM, Nyffeler RW. Experimental rotator cuff repair: a preliminary study. J Bone Joint Surg Am. 1999; 81(9):1281–1290. PMID: 10505524.
Article6. Kovacevic D, Rodeo SA. Biological augmentation of rotator cuff tendon repair. Clin Orthop Relat Res. 2008; 466(3):622–633. PMID: 18264850.
Article7. Cheung EV, Silverio L, Sperling JW. Strategies in biologic augmentation of rotator cuff repair: a review. Clin Orthop Relat Res. 2010; 468(6):1476–1484. PMID: 20352390.
Article8. Murphy MB, Blashki D, Buchanan RM, et al. Adult and umbilical cord blood-derived platelet-rich plasma for mesenchymal stem cell proliferation, chemotaxis, and cryopreservation. Biomaterials. 2012; 33(21):5308–5316. PMID: 22542609.
Article9. Xie X, Wang Y, Zhao C, et al. Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration. Biomaterials. 2012; 33(29):7008–7018. PMID: 22818985.
Article10. Kim HJ, Nam HW, Hur CY, et al. The effect of platelet rich plasma from bone marrow aspirate with added bone morphogenetic protein-2 on the Achilles tendon-bone junction in rabbits. Clin Orthop Surg. 2011; 3(4):325–331. PMID: 22162796.
Article11. Nishimoto S, Fukuda K, Kawai K, et al. Supplementation of bone marrow aspirate-derived platelet-rich plasma for treating radiation-induced ulcer after cardiac fluoroscopic procedures: a preliminary report. Indian J Plast Surg. 2012; 45(1):109–114. PMID: 22754164.
Article12. Yun JH, Han SH, Choi SH, et al. Effects of bone marrow-derived mesenchymal stem cells and platelet-rich plasma on bone regeneration for osseointegration of dental implants: preliminary study in canine three-wall intrabony defects. J Biomed Mater Res B Appl Biomater. 2014; 102(5):1021–1030. PMID: 24307497.
Article13. Oh JH, Chung SW, Kim SH, Chung JY, Kim JY. effect of the adipose-derived stem cell for the improvement of fatty degeneration and rotator cuff healing in rabbit model. J Shoulder Elbow Surg. 2014; 23(4):445–455. PMID: 24129058.
Article14. Uhthoff HK, Seki M, Backman DS, Trudel G, Himori K, Sano H. Tensile strength of the supraspinatus after reimplantation into a bony trough: an experimental study in rabbits. J Shoulder Elbow Surg. 2002; 11(5):504–509. PMID: 12378172.
Article15. Rubino LJ, Stills HF Jr, Sprott DC, Crosby LA. Fatty infiltration of the torn rotator cuff worsens over time in a rabbit model. Arthroscopy. 2007; 23(7):717–722. PMID: 17637406.
Article16. Itoigawa Y, Kishimoto KN, Sano H, Kaneko K, Itoi E. Molecular mechanism of fatty degeneration in rotator cuff muscle with tendon rupture. J Orthop Res. 2011; 29(6):861–866. PMID: 21246616.
Article17. Hirose K, Kondo S, Choi HR, Mishima S, Iwata H, Ishiguro N. Spontaneous healing process of a supraspinatus tendon tear in rabbits. Arch Orthop Trauma Surg. 2004; 124(6):374–377. PMID: 15156330.
Article18. Chung SW, Song BW, Kim YH, Park KU, Oh JH. Effect of platelet-rich plasma and porcine dermal collagen graft augmentation for rotator cuff healing in a rabbit model. Am J Sports Med. 2013; 41(12):2909–2918. PMID: 24047553.
Article19. Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009; 37(11):2259–2272. PMID: 19875361.20. Zhong W, Sumita Y, Ohba S, et al. In vivo comparison of the bone regeneration capability of human bone marrow concentrates vs. platelet-rich plasma. PLoS One. 2012; 7(7):e40833. PMID: 22808272.
Article21. Gulotta LV, Kovacevic D, Ehteshami JR, Dagher E, Packer JD, Rodeo SA. Application of bone marrow-derived mesenchymal stem cells in a rotator cuff repair model. Am J Sports Med. 2009; 37(11):2126–2133. PMID: 19684297.
Article22. Geuze RE, Kruyt MC, Verbout AJ, Alblas J, Dhert WJ. Comparing various off-the-shelf methods for bone tissue engineering in a large-animal ectopic implantation model: bone marrow, allogeneic bone marrow stromal cells, and platelet gel. Tissue Eng Part A. 2008; 14(8):1435–1443. PMID: 18601585.
Article23. Hoffmann A, Gross G. Tendon and ligament engineering: from cell biology to in vivo application. Regen Med. 2006; 1(4):563–574. PMID: 17465850.
Article24. Okamoto N, Kushida T, Oe K, Umeda M, Ikehara S, Iida H. Treating Achilles tendon rupture in rats with bone-marrow-cell transplantation therapy. J Bone Joint Surg Am. 2010; 92(17):2776–2784. PMID: 21123607.
Article25. Lyras DN, Kazakos K, Verettas D, et al. The effect of platelet-rich plasma gel in the early phase of patellar tendon healing. Arch Orthop Trauma Surg. 2009; 129(11):1577–1582. PMID: 19621231.
Article26. Aspenberg P, Virchenko O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop Scand. 2004; 75(1):93–99. PMID: 15022816.
Article27. Bosch G, van Schie HT, de Groot MW, et al. Effects of platelet-rich plasma on the quality of repair of mechanically induced core lesions in equine superficial digital flexor tendons: a placebo-controlled experimental study. J Orthop Res. 2010; 28(2):211–217. PMID: 19714688.
Article28. Zhang J, Wang JH. Platelet-rich plasma releasate promotes differentiation of tendon stem cells into active tenocytes. Am J Sports Med. 2010; 38(12):2477–2486. PMID: 20802092.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current and Future Strategies to Enhance Healing at the Tendon-To-Bone Interface of a Rotator Cuff Tear
- Usefulness of Multiphase Scaffolds for Improving Tendon to Bone Healing for Rotator Cuff Tears in Shoulder
- Controversy in Pathophysiology of Rotator Cuff Tear: Degenerative Tear
- Current Research on the Influence of Statin Treatment on Rotator Cuff Healing
- Hyperlipidemia and Rotator Cuff Tears: Exploring Mechanisms and Effective Treatment