J Periodontal Implant Sci.
2018 Feb;48(1):60-68. 10.5051/jpis.2018.48.1.60.
Porphyromonas gingivalis accelerates atherosclerosis through oxidation of high-density lipoprotein
- Affiliations
-
- 1Department of Periodontology, Pusan National University School of Dentistry, Yangsan, Korea. joojy@pusan.ac.kr
- 2Department of Oral Physiology, Institute of Translational Dental Sciences, Pusan National University School of Dentistry, Yangsan, Korea.
- 3Dental Clinic Center, Pusan National University Hospital, Busan, Korea.
- 4Department of Periodontology and Dental Research Institute, Pusan National University Dental Hospital, Yangsan, Korea.
- KMID: 2405410
- DOI: http://doi.org/10.5051/jpis.2018.48.1.60
Abstract
- PURPOSE
The aim of this study was to evaluate the ability of Porphyromonas gingivalis (P. gingivalis) to induce oxidation of high-density lipoprotein (HDL) and to determine whether the oxidized HDL induced by P. gingivalis exhibited altered antiatherogenic function or became proatherogenic.
METHODS
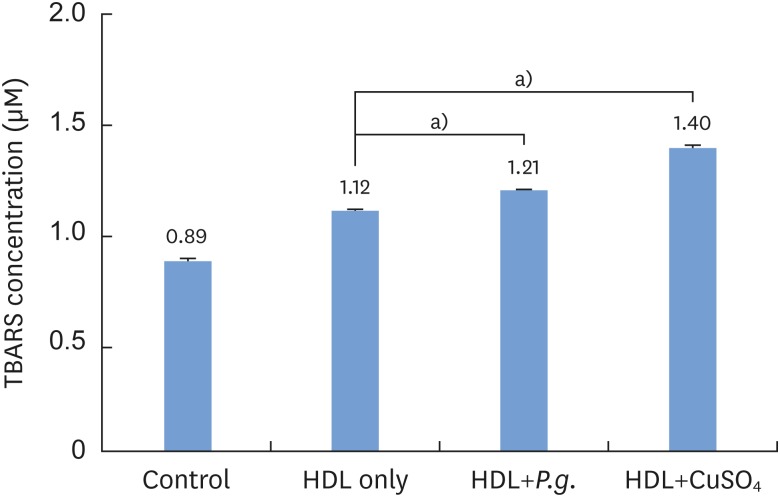
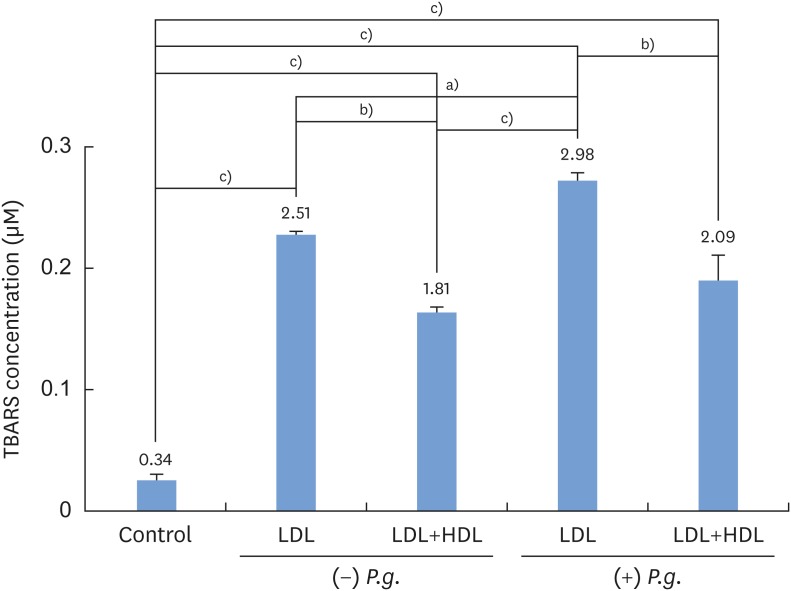
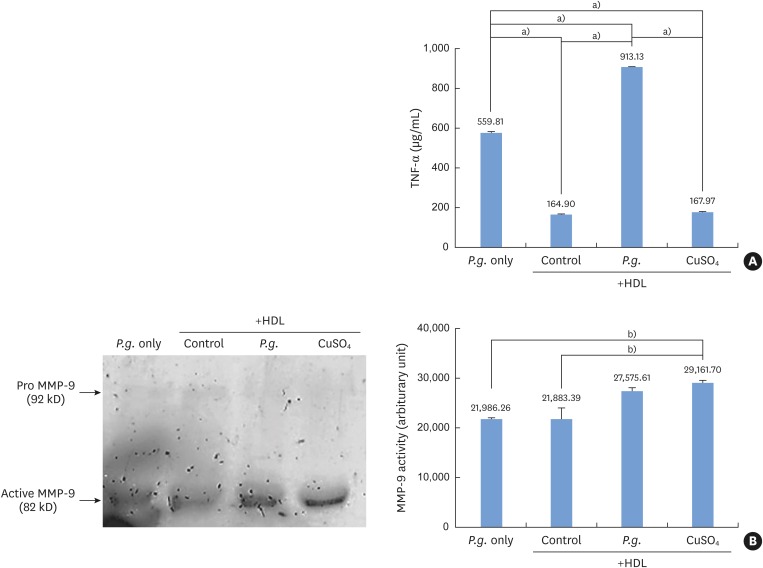
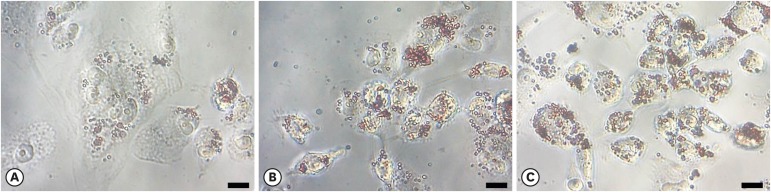
P. gingivalis and THP-1 monocytes were cultured, and the extent of HDL oxidation induced by P. gingivalis was evaluated by a thiobarbituric acid-reactive substances (TBARS) assay. To evaluate the altered antiatherogenic and proatherogenic properties of P. gingivalis-treated HDL, lipid oxidation was quantified by the TBARS assay, and tumor necrosis factor alpha (TNF-α) levels and the gelatinolytic activity of matrix metalloproteinase (MMP)-9 were also measured. After incubating macrophages with HDL and P. gingivalis, Oil Red O staining was performed to examine foam cells.
RESULTS
P. gingivalis induced HDL oxidation. The HDL treated by P. gingivalis did not reduce lipid oxidation and may have enhanced the formation of MMP-9 and TNF-α. P. gingivalis-treated macrophages exhibited more lipid aggregates than untreated macrophages.
CONCLUSIONS
P. gingivalis induced HDL oxidation, impairing the atheroprotective function of HDL and making it proatherogenic by eliciting a proinflammatory response through its interaction with monocytes/macrophages.
Keyword
MeSH Terms
-
Atherosclerosis*
Cardiovascular Diseases
Cholesterol
Foam Cells
Lipoproteins*
Macrophages
Monocytes
Periodontitis
Porphyromonas gingivalis*
Porphyromonas*
Thiobarbituric Acid Reactive Substances
Tumor Necrosis Factor-alpha
Cholesterol
Lipoproteins
Thiobarbituric Acid Reactive Substances
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Genco R, Offenbacher S, Beck J. Periodontal disease and cardiovascular disease: epidemiology and possible mechanisms. J Am Dent Assoc. 2002; 133:14S–22S. PMID: 12085720.2. Koizumi Y, Kurita-Ochiai T, Oguchi S, Yamamoto M. Nasal immunization with Porphyromonas gingivalis outer membrane protein decreases P. gingivalis-induced atherosclerosis and inflammation in spontaneously hyperlipidemic mice. Infect Immun. 2008; 76:2958–2965. PMID: 18426881.3. Muhlestein JB, Anderson JL, Hammond EH, Zhao L, Trehan S, Schwobe EP, et al. Infection with Chlamydia pneumoniae accelerates the development of atherosclerosis and treatment with azithromycin prevents it in a rabbit model. Circulation. 1998; 97:633–636. PMID: 9495296.4. Lösche W, Karapetow F, Pohl A, Pohl C, Kocher T. Plasma lipid and blood glucose levels in patients with destructive periodontal disease. J Clin Periodontol. 2000; 27:537–541. PMID: 10959778.
Article5. Laurila A, Bloigu A, Näyhä S, Hassi J, Leinonen M, Saikku P. Chronic Chlamydia pneumoniae infection is associated with a serum lipid profile known to be a risk factor for atherosclerosis. Arterioscler Thromb Vasc Biol. 1997; 17:2910–2913. PMID: 9409275.6. Laurila A, Bloigu A, Näyhä S, Hassi J, Leinonen M, Saikku P. Association of Helicobacter pylori infection with elevated serum lipids. Atherosclerosis. 1999; 142:207–210. PMID: 9920523.7. Pussinen PJ, Jauhiainen M, Vilkuna-Rautiainen T, Sundvall J, Vesanen M, Mattila K, et al. Periodontitis decreases the antiatherogenic potency of high density lipoprotein. J Lipid Res. 2004; 45:139–147. PMID: 13130123.
Article8. Steinberg D. Low density lipoprotein oxidation and its pathobiological significance. J Biol Chem. 1997; 272:20963–20966. PMID: 9261091.
Article9. Mertens A, Holvoet P. Oxidized LDL and HDL: antagonists in atherothrombosis. FASEB J. 2001; 15:2073–2084. PMID: 11641234.
Article10. Rosenson RS, Brewer HB Jr, Ansell BJ, Barter P, Chapman MJ, Heinecke JW, et al. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat Rev Cardiol. 2016; 13:48–60. PMID: 26323267.
Article11. Khovidhunkit W, Shigenaga JK, Moser AH, Feingold KR, Grunfeld C. Cholesterol efflux by acute-phase high density lipoprotein: role of lecithin:cholesterol acyltransferase. J Lipid Res. 2001; 42:967–975. PMID: 11369805.
Article12. Kameda T, Ohkawa R, Yano K, Usami Y, Miyazaki A, Matsuda K, et al. Effects of myeloperoxidase-induced oxidation on antiatherogenic functions of high-density lipoprotein. J Lipids. 2015; 2015:592594. PMID: 26257958.
Article13. Florentin M, Liberopoulos EN, Wierzbicki AS, Mikhailidis DP. Multiple actions of high-density lipoprotein. Curr Opin Cardiol. 2008; 23:370–378. PMID: 18520722.
Article14. Francis GA. High density lipoprotein oxidation: in vitro susceptibility and potential in vivo consequences. Biochim Biophys Acta. 2000; 1483:217–235. PMID: 10634938.15. Barter PJ, Baker PW, Rye KA. Effect of high-density lipoproteins on the expression of adhesion molecules in endothelial cells. Curr Opin Lipidol. 2002; 13:285–288. PMID: 12045398.
Article16. Maekawa T, Takahashi N, Tabeta K, Aoki Y, Miyashita H, Miyauchi S, et al. Chronic oral infection with Porphyromonas gingivalis accelerates atheroma formation by shifting the lipid profile. PLoS One. 2011; 6:e20240. PMID: 21625524.17. Miyakawa H, Honma K, Qi M, Kuramitsu HK. Interaction of Porphyromonas gingivalis with low-density lipoproteins: implications for a role for periodontitis in atherosclerosis. J Periodontal Res. 2004; 39:1–9. PMID: 14687221.18. Joo JY, Cha GS, Chung J, Lee JY, Kim SJ, Choi J. Peptide 19 of Porphyromonas gingivalis heat shock protein is a potent inducer of low-density lipoprotein oxidation. J Periodontol. 2017; 88:e58–e64. PMID: 27712463.19. Soumyarani VS, Jayakumari N. Oxidized HDL induces cytotoxic effects: implications for atherogenic mechanism. J Biochem Mol Toxicol. 2014; 28:481–489. PMID: 25044446.
Article20. Soumyarani VS, Jayakumari N. Oxidatively modified high density lipoprotein promotes inflammatory response in human monocytes-macrophages by enhanced production of ROS, TNF-α, MMP-9, and MMP-2. Mol Cell Biochem. 2012; 366:277–285. PMID: 22527933.
Article21. Persson J, Nilsson J, Lindholm MW. Interleukin-1beta and tumour necrosis factor-alpha impede neutral lipid turnover in macrophage-derived foam cells. BMC Immunol. 2008; 9:70. PMID: 19032770.
Article22. Jones CB, Sane DC, Herrington DM. Matrix metalloproteinases: a review of their structure and role in acute coronary syndrome. Cardiovasc Res. 2003; 59:812–823. PMID: 14553821.
Article23. Gough PJ, Gomez IG, Wille PT, Raines EW. Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J Clin Invest. 2006; 116:59–69. PMID: 16374516.
Article24. Ren J, Jin W, Chen H. oxHDL decreases the expression of CD36 on human macrophages through PPARgamma and p38 MAP kinase dependent mechanisms. Mol Cell Biochem. 2010; 342:171–181. PMID: 20458524.25. Nakajima T, Origuchi N, Matsunaga T, Kawai S, Hokari S, Nakamura H, et al. Localization of oxidized HDL in atheromatous plaques and oxidized HDL binding sites on human aortic endothelial cells. Ann Clin Biochem. 2000; 37:179–186. PMID: 10735361.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- T-and cross-reactive B-cell epitopes of Porphyromonas gingivalis and human heat shock protein 60 in atherosclerosis
- Environmental factors regulating the expression of Porphyromonas gingivalis heat shock protein
- The relation between metabolic acid and the virulence of porphyromonas gingivalis serotype b
- Epitope specificity of Porphyromonas gingivalis heat shock protein for T-cell and/or B-cell in human atherosclerosis
- Fusobacterium nucleatum modulates serum binding to Porphyromonas gingivalis biofilm