Cancer Res Treat.
2018 Jan;50(1):60-70. 10.4143/crt.2016.533.
Magnetic Resonance Imaging for Colorectal Cancer Metastasis to the Liver: Comparative Effectiveness Research for the Choice of Contrast Agents
- Affiliations
-
- 1Department of Radiology, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. radpms@yuhs.ac
- 2Department of Radiology, Yonsei Biomedical Research Institute, Research Institute of Radiological Science, Seoul, Korea.
- 3Department of Radiology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 4Department of Radiology and Research Institute of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 5Department of Surgery, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2403475
- DOI: http://doi.org/10.4143/crt.2016.533
Abstract
- PURPOSE
This study was conducted to compare the diagnostic performance and early recurrence rate between gadoxetic acid-enhanced magnetic resonance imaging (Gd-EOB-MRI) and magnetic resonance imaging (MRI) with extracellular contrast agent (ECA-MRI) for evaluating hepatic lesions in colorectal cancer.
MATERIALS AND METHODS
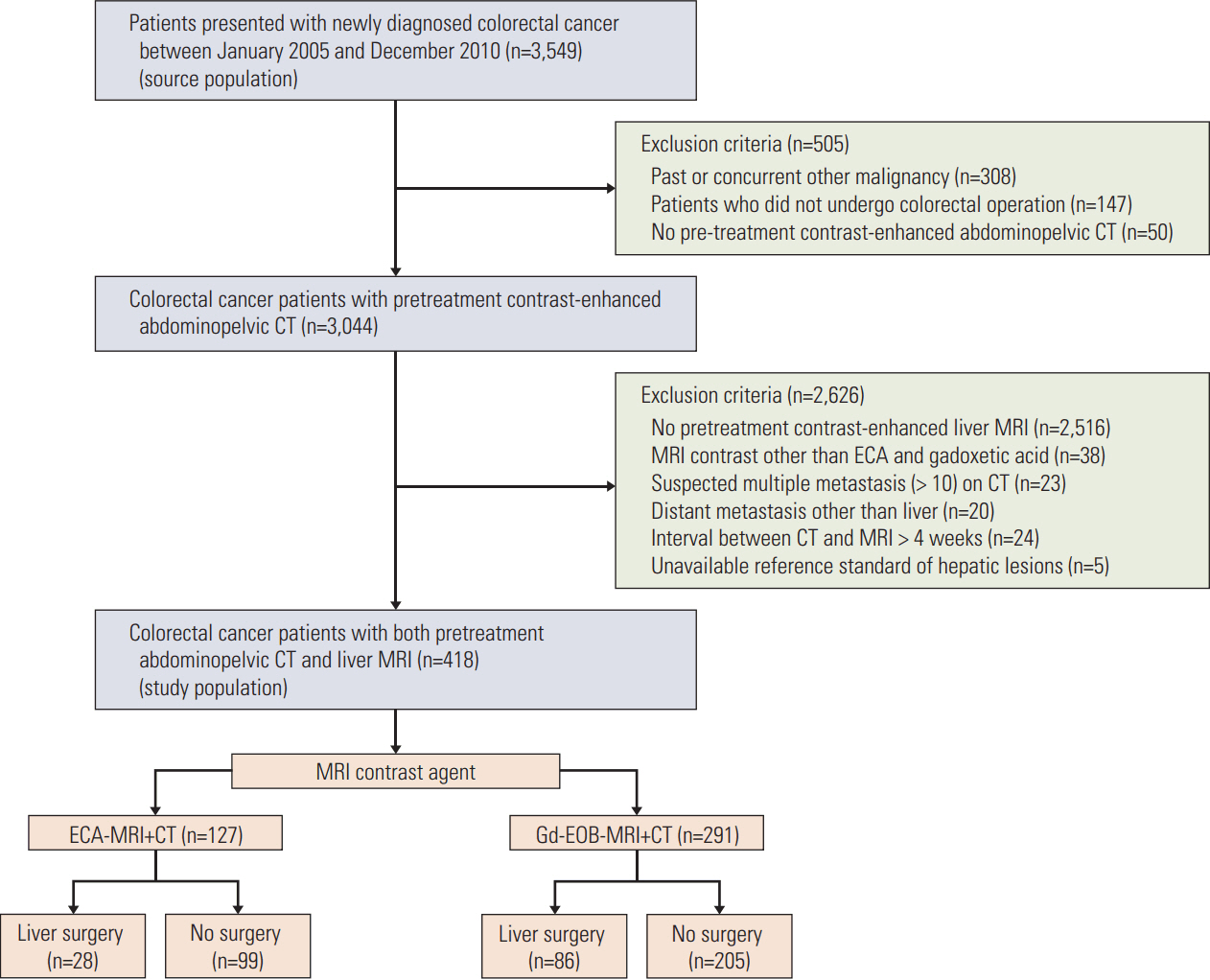
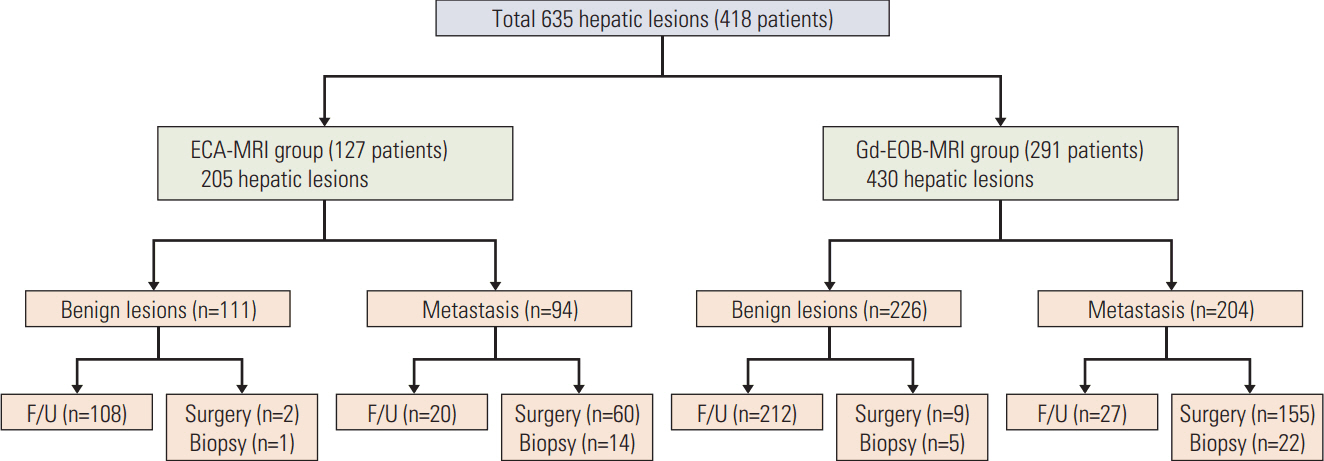
Between 2005 and 2010, 418 colorectal cancer patients with both preoperative computed tomography (CT) and liver MRI were retrospectively reviewed. Image analysis was based on initial radiologic reports, and diagnostic performance was assessed based on the area under the receiver operating characteristic curve (AUROC). The early intrahepatic recurrence rate within 6 months was then evaluated.
RESULTS
Overall, 291 and 127 patients underwent Gd-EOB-MRI and ECA-MRI, respectively. The AUROCs were not significantly different between Gd-EOB-MRI (0.990; 95% CI, 0.980 to 0.999) and ECA-MRI (0.985; 95% CI, 0.968 to 1.000; p=0.836). When compared with CT alone, ECA-MRI detected additional 21 lesions in 14 patients (14/127, 11.0%), whereas Gd-EOB-MRI detected 56 lesions in 33 patients (33/291, 11.3%) without a significant difference between two MRI groups (p=0.331). The early recurrence rate in the ECA-MRI (28.6%) was significantly higher than that in the Gd-EOB-MRI (11.6%) for patients who underwent hepatic resection (p=0.031).
CONCLUSION
Gd-EOB-MRI is potentially better than ECA-MRI for decreasing the early intrahepatic recurrence rate, although the two MRI modalities showed comparable diagnostic performance in colorectal cancer patients.
MeSH Terms
Figure
Cited by 1 articles
-
Incremental Role of Pancreatic Magnetic Resonance Imaging after Staging Computed Tomography to Evaluate Patients with Pancreatic Ductal Adenocarcinoma
Hye Jin Kim, Mi-Suk Park, Jin Yong Lee, Kyunghwa Han, Yong Eun Chung, Jin-Young Choi, Myeong-Jin Kim, Chang Moo Kang
Cancer Res Treat. 2019;51(1):24-33. doi: 10.4143/crt.2017.404.
Reference
-
References
1. Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006; 244:254–9.
Article2. Norstein J, Silen W. Natural history of liver metastases from colorectal carcinoma. J Gastrointest Surg. 1997; 1:398–407.
Article3. Pawlik TM, Choti MA. Surgical therapy for colorectal metastases to the liver. J Gastrointest Surg. 2007; 11:1057–77.
Article4. Vigano L, Russolillo N, Ferrero A, Langella S, Sperti E, Capussotti L. Evolution of long-term outcome of liver resection for colorectal metastases: analysis of actual 5-year survival rates over two decades. Ann Surg Oncol. 2012; 19:2035–44.
Article5. National Comprehensive Cancer Network. Clinical practice guidelines in oncology: colon cancer [Internet]. Fort Washington, PA: National Comprehensive Cancer Network;2014. [cited 2015 Oct 21]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.6. National Comprehensive Cancer Network. Clinical practice guidelines in oncology: rectal cancer [Internet]. Fort Washington, PA: National Comprehensive Cancer Network;2014. [cited 2015 Oct 21]. Available from: http://www.nccn.org/professionals/physicial_gls/pdf/rectal.pdf.7. Tirumani SH, Kim KW, Nishino M, Howard SA, Krajewski KM, Jagannathan JP, et al. Update on the role of imaging in management of metastatic colorectal cancer. Radiographics. 2014; 34:1908–28.
Article8. Kulemann V, Schima W, Tamandl D, Kaczirek K, Gruenberger T, Wrba F, et al. Preoperative detection of colorectal liver metastases in fatty liver: MDCT or MRI? Eur J Radiol. 2011; 79:e1–6.
Article9. Zech CJ, Korpraphong P, Huppertz A, Denecke T, Kim MJ, Tanomkiat W, et al. Randomized multicentre trial of gadoxetic acid-enhanced MRI versus conventional MRI or CT in the staging of colorectal cancer liver metastases. Br J Surg. 2014; 101:613–21.
Article10. Han K, Park SH, Kim KW, Kim HJ, Lee SS, Kim JC, et al. Use of liver magnetic resonance imaging after standard staging abdominopelvic computed tomography to evaluate newly diagnosed colorectal cancer patients. Ann Surg. 2015; 261:480–6.
Article11. Kim HJ, Lee SS, Byun JH, Kim JC, Yu CS, Park SH, et al. Incremental value of liver MR imaging in patients with potentially curable colorectal hepatic metastasis detected at CT: a prospective comparison of diffusion-weighted imaging, gadoxetic acid-enhanced MR imaging, and a combination of both MR techniques. Radiology. 2015; 274:712–22.
Article12. Niekel MC, Bipat S, Stoker J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology. 2010; 257:674–84.
Article13. Floriani I, Torri V, Rulli E, Garavaglia D, Compagnoni A, Salvolini L, et al. Performance of imaging modalities in diagnosis of liver metastases from colorectal cancer: a systematic review and meta-analysis. J Magn Reson Imaging. 2010; 31:19–31.
Article14. Jhaveri K, Cleary S, Audet P, Balaa F, Bhayana D, Burak K, et al. Consensus statements from a multidisciplinary expert panel on the utilization and application of a liver-specific MRI contrast agent (gadoxetic acid). AJR Am J Roentgenol. 2015; 204:498–509.
Article15. Merkle EM, Zech CJ, Bartolozzi C, Bashir MR, Ba-Ssalamah A, Huppertz A, et al. Consensus report from the 7th International Forum for Liver Magnetic Resonance Imaging. Eur Radiol. 2016; 26:674–82.
Article16. Kim YK, Kim CS, Han YM, Park G. Detection of small hepatocellular carcinoma: can gadoxetic acid-enhanced magnetic resonance imaging replace combining gadopentetate dimeglumine-enhanced and superparamagnetic iron oxide-enhanced magnetic resonance imaging? Invest Radiol. 2010; 45:740–6.17. Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988; 16:1141–54.
Article18. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999; 94:496–509.
Article19. Chen L, Zhang J, Zhang L, Bao J, Liu C, Xia Y, et al. Meta-analysis of gadoxetic acid disodium (Gd-EOB-DTPA)-enhanced magnetic resonance imaging for the detection of liver metastases. PLoS One. 2012; 7:e48681.
Article20. Vilgrain V, Esvan M, Ronot M, Caumont-Prim A, Aube C, Chatellier G. A meta-analysis of diffusion-weighted and gadoxetic acid-enhanced MR imaging for the detection of liver metastases. Eur Radiol. 2016; 26:4595–615.
Article21. Mueller GC, Hussain HK, Carlos RC, Nghiem HV, Francis IR. Effectiveness of MR imaging in characterizing small hepatic lesions: routine versus expert interpretation. AJR Am J Roentgenol. 2003; 180:673–80.22. Pilgrim CH, Thomson BN, Banting S, Phillips WA, Michael M. The developing clinical problem of chemotherapy-induced hepatic injury. ANZ J Surg. 2012; 82:23–9.
Article23. Robinson PJ. The effects of cancer chemotherapy on liver imaging. Eur Radiol. 2009; 19:1752–62.
Article24. Vigano L, Capussotti L, Lapointe R, Barroso E, Hubert C, Giuliante F, et al. Early recurrence after liver resection for colorectal metastases: risk factors, prognosis, and treatment. A LiverMetSurvey-based study of 6,025 patients. Ann Surg Oncol. 2014; 21:1276–86.
Article25. Takahashi S, Konishi M, Kinoshita T, Gotohda N, Kato Y, Saito N, et al. Predictors for early recurrence after hepatectomy for initially unresectable colorectal liver metastasis. J Gastrointest Surg. 2013; 17:939–48.
Article26. Malik HZ, Gomez D, Wong V, Al-Mukthar A, Toogood GJ, Lodge JP, et al. Predictors of early disease recurrence following hepatic resection for colorectal cancer metastasis. Eur J Surg Oncol. 2007; 33:1003–9.
Article27. Takahashi S, Konishi M, Nakagohri T, Gotohda N, Saito N, Kinoshita T. Short time to recurrence after hepatic resection correlates with poor prognosis in colorectal hepatic metastasis. Jpn J Clin Oncol. 2006; 36:368–75.
Article28. Tan MC, Butte JM, Gonen M, Kemeny N, Fong Y, Allen PJ, et al. Prognostic significance of early recurrence: a conditional survival analysis in patients with resected colorectal liver metastasis. HPB (Oxford). 2013; 15:803–13.
Article29. Zhou XH. Correcting for verification bias in studies of a diagnostic test's accuracy. Stat Methods Med Res. 1998; 7:337–53.
Article30. Begg CB, Greenes RA. Assessment of diagnostic tests when disease verification is subject to selection bias. Biometrics. 1983; 39:207–15.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Detection of Hepatic Metastases from Colorectal Cancer: A Comparative Study between the SPIO-enhanced MR and Intraoperative Ultrasound
- MR Contrast Agents and Molecular Imaging
- Dynamic Contrast-Enhanced Magnetic Resonance Imaging as a Surrogate Biomarker for Bevacizumab in Colorectal Cancer Liver Metastasis: A Single-Arm, Exploratory Trial
- Molecular MR Imaging
- Use of Abbreviated Magnetic Resonance Imaging in Breast Cancer Screening