J Vet Sci.
2018 Jan;19(1):107-115. 10.4142/jvs.2018.19.1.107.
Tibial dyschondroplasia is closely related to suppression of expression of hypoxia-inducible factors 1α, 2α, and 3α in chickens
- Affiliations
-
- 1College of Veterinary Medicine, Huazhong Agricultural University, Wuhan 430070, China. lijk210@sina.com
- 2Laboratory of Detection and Monitoring of Highland Animal Disease, Tibet Agriculture and Animal Husbandry College, Linzhi 860000, Tibet, China.
- 3Animal Science Department, Wenzhou Vocational College of Science and Technology, Wenzhou 325006, China.
- KMID: 2402701
- DOI: http://doi.org/10.4142/jvs.2018.19.1.107
Abstract
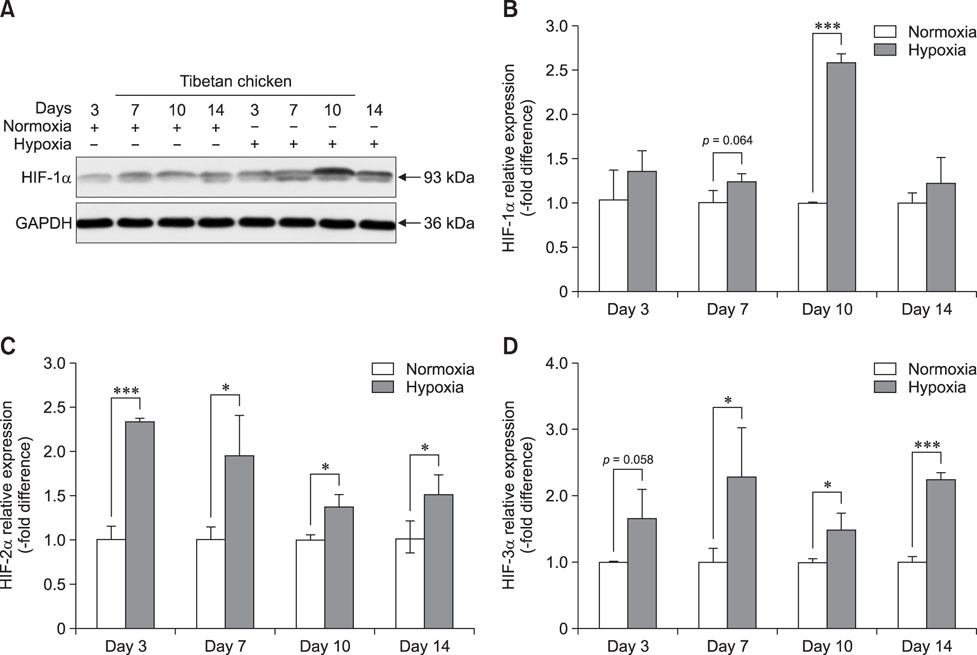
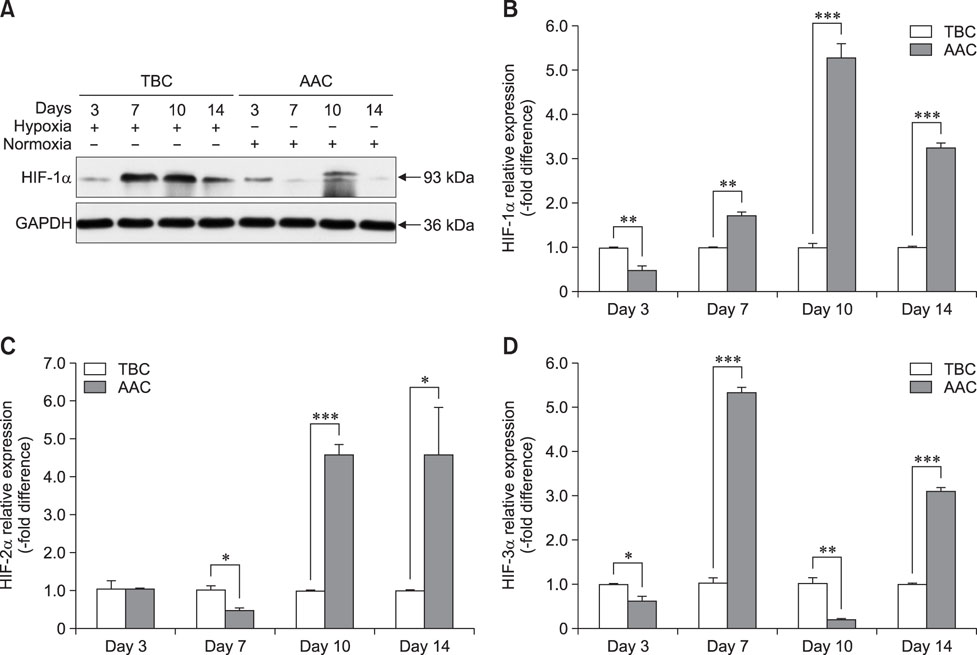
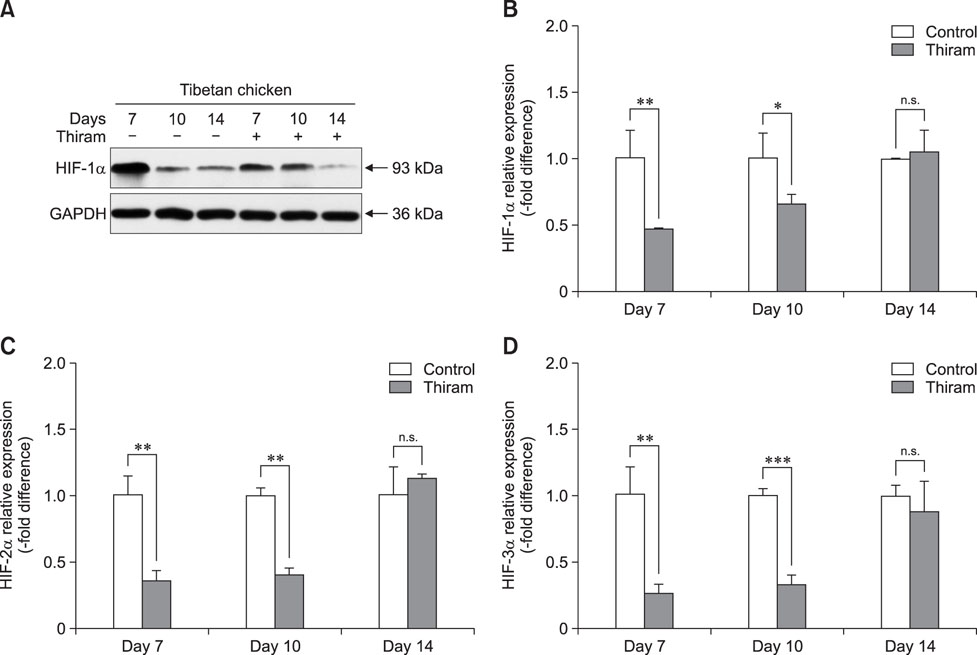
- Tibial dyschondroplasia (TD) cases has not been reported in Tibetan chickens (TBCs), but it is commonly seen in commercial broilers characterized by lameness. The underlying mechanism remains unclear. Hypoxia-inducible factors (HIFs) are important regulators of cellular adaptation to hypoxic conditions. In this study, we investigated the role of HIF-1α, -2α, and -3α in hypoxia and thiram-induced TD and their effect on tibial growth plate development in Arbor Acres chickens (AACs) and TBCs. RNA and protein expression levels of HIF-1α, -2α, and -3α were determined by using quantitative reverse transcriptase polymerase chain reaction and western blotting analyses, respectively. Interestingly, the results showed that HIF-1α, -2α, and -3α expressions in the tibial growth plate of TBCs were upregulated by hypoxia and the change was more significant in TBCs than in AACs. However, these factors were downregulated in thiram-induced TD. To further clarify the effect of thiram on tibial growth plate in commercial broilers, AACs were observed to exhibit more pronounced changes in their growth plate that that in TBCs. Taken together, these results demonstrate that HIF-1α, -2α, and -3α may be important in tibial growth plate development and in the prevention of TD. The present study contributes novel insights on a therapeutic target for poultry TD.
Keyword
MeSH Terms
Figure
Reference
-
1. Befani CD, Vlachostergios PJ, Hatzidaki E, Patrikidou A, Bonanou S, Simos G, Papandreou CN, Liakos P. Bortezomib represses HIF-1α protein expression and nuclear accumulation by inhibiting both PI3K/Akt/TOR and MAPK pathways in prostate cancer cells. J Mol Med (Berl). 2012; 90:45–54.
Article2. Bohensky J, Terkhorn SP, Freeman TA, Adams CS, Garcia JA, Shapiro IM, Srinivas V. Regulation of autophagy in human and murine cartilage: hypoxia-inducible factor 2 suppresses chondrocyte autophagy. Arthritis Rheum. 2009; 60:1406–1415.
Article3. Chen J, Chen AY, Huang H, Ye X, Rollyson WD, Perry HE, Brown KC, Rojanasakul Y, Rankin GO, Dasgupta P, Chen YC. The flavonoid nobiletin inhibits tumor growth and angiogenesis of ovarian cancers via the Akt pathway. Int J Oncol. 2015; 46:2629–2638.
Article4. Cheng KJ, Bao YY, Zhou SH. The role of hypoxia inducible factor in nasal inflammations. Eur Rev Med Pharmacol Sci. 2016; 20:5067–5076.5. Groves PJ, Muir WI. Earlier hatching time predisposes Cobb broiler chickens to tibial dyschondroplasia. Animal. 2017; 11:112–120.
Article6. Huang S, Zhang L, Rehman MU, Iqbal MK, Lan Y, Mehmood K, Zhang H, Qiu G, Nabi F, Yao W, Wang M, Li J. High altitude hypoxia as a factor that promotes tibial growth plate development in broiler chickens. PLoS One. 2017; 12:e0173698.
Article7. Huang SC, Rehman MU, Lan YF, Qiu G, Zhang H, Iqbal MK, Luo HQ, Mehmood K, Zhang LH, Li JK. Tibial dyschondroplasia is highly associated with suppression of tibial angiogenesis through regulating the HIF-1α/VEGF/VEGFR signaling pathway in chickens. Sci Rep. 2017; 7:9089.
Article8. Lefebvre V, Smits P. Transcriptional control of chondrocyte fate and differentiation. Birth Defects Res C Embryo Today. 2005; 75:200–212.
Article9. Li H, Guo S, Ren Y, Wang D, Yu H, Li W, Zhao X, Chang Z. VEGF189 expression is highly related to adaptation of the plateau pika (Ochotona curzoniae) inhabiting high altitudes. High Alt Med Biol. 2013; 14:395–404.
Article10. Li J, Bi D, Pan S, Zhang Y. Effect of diet with thiram on liver antioxidant capacity and tibial dyschondroplasia in broilers. Br Poult Sci. 2007; 48:724–728.
Article11. Li L, Qu Y, Jin X, Guo XQ, Wang Y, Qi L, Yang J, Zhang P, Li LZ. Protective effect of salidroside against bone loss via hypoxia-inducible factor-1α pathway-induced angiogenesis. Sci Rep. 2016; 6:32131.
Article12. Li M, Zhao C. Study on Tibetan Chicken embryonic adaptability to chronic hypoxia by revealing differential gene expression in heart tissue. Sci China C Life Sci. 2009; 52:284–295.
Article13. Markway BD, Cho H, Zilberman-Rudenko J, Holden P, McAlinden A, Johnstone B. Hypoxia-inducible factor 3-alpha expression is associated with the stable chondrocyte phenotype. J Orthop Res. 2015; 33:1561–1570.
Article14. Nabi F, Shahzad M, Liu J, Li K, Han Z, Zhang D, Iqbal MK, Li J. Hsp90 inhibitor celastrol reinstates growth plate angiogenesis in thiram-induced tibial dyschondroplasia. Avian Pathol. 2016; 45:187–193.
Article15. Pelicia K, Aparecido Jr IM, Garcia EA, Molino AB, Santos GC, Berto DA, Vieira-Filho JA, Murakami ESM, Montenegro AT, Silva AM. Evaluation of a radiographic method to detect tibial dyschondroplasia lesions in broilers. Braz J Poultry Sci. 2012; 14:129–135.
Article16. Rath NC, Kannan L, Pillai PB, Huff WE, Huff GR, Horst RL, Emmert JL. Evaluation of the efficacy of vitamin D3 or its metabolites on thiram-induced tibial dyschondroplasia in chickens. Res Vet Sci. 2007; 83:244–250.
Article17. Schipani E, Ryan HE, Didrickson S, Kobayashi T, Knight M, Johnson RS. Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev. 2001; 15:2865–2876.
Article18. Shahzad M, Gao J, Qin P, Liu J, Wang Z, Zhang D, Li J. Expression of genes encoding matrilin-3 and cyclin-I during the impairment and recovery of chicken growth plate in tibial dyschondroplasia. Avian Dis. 2014; 58:468–473.
Article19. Shapiro F. Bone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblasts. Eur Cell Mater. 2008; 15:53–76.
Article20. Stewart AJ, Houston B, Farquharson C. Elevated expression of hypoxia inducible factor-2alpha in terminally differentiating growth plate chondrocytes. J Cell Physiol. 2006; 206:435–440.
Article21. Su Y, Li D, Gaur U, Wang Y, Wu N, Chen B, Xu Z, Yin H, Hu Y, Zhu Q. Genetic diversity of bitter taste receptor gene family in Sichuan domestic and Tibetan chicken populations. J Genet. 2016; 95:675–681.
Article22. Takagi H, Koyama S, Seike H, Oh H, Otani A, Matsumura M, Honda Y. Potential role of the angiopoietin/tie2 system in ischemia-induced retinal neovascularization. Invest Ophthalmol Vis Sci. 2003; 44:393–402.
Article23. Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995; 92:5510–5514.
Article24. Wu J, Ke X, Fu W, Gao X, Zhang H, Wang W, Ma N, Zhao M, Hao X, Zhang Z. Inhibition of hypoxia-induced retinal angiogenesis by specnuezhenide, an effective constituent of Ligustrum lucidum Ait., through suppression of the HIF-1α/VEGF signaling pathway. Molecules. 2016; 21:E1756.25. Xue X, Ramakrishnan S, Anderson E, Taylor M, Zimmermann EM, Spence JR, Huang S, Greenson JK, Shah YM. Endothelial PAS domain protein 1 activates the inflammatory response in the intestinal epithelium to promote colitis in mice. Gastroenterology. 2013; 145:831–841.
Article26. Yamakawa M, Liu LX, Belanger AJ, Date T, Kuriyama T, Goldberg MA, Cheng SH, Gregory RJ, Jiang C. Expression of angiopoietins in renal epithelial and clear cell carcinoma cells: regulation by hypoxia and participation in angiogenesis. Am J Physiol Renal Physiol. 2004; 287:F649–F657.
Article27. Yang T, Yao Q, Cao F, Liu Q, Liu B, Wang XH. Silver nanoparticles inhibit the function of hypoxia-inducible factor-1 and target genes: insight into the cytotoxicity and antiangiogenesis. Int J Nanomedicine. 2016; 11:6679–6692.
Article28. Yang YZ, Bai Y, Dong XX, Zhang JB, Fang M. Expression analysis of HIF-1α and HIF-2α genes in tibetan chicken under normoxia and hypoxia. J Anim Vet Adv. 2012; 10:2172–2175.
Article29. Yin R, Yuan L, Ping L, Hu L. Neonatal bronchopulmonary dysplasia increases neuronal apoptosis in the hippocampus through the HIF-1α and p53 pathways. Respir Physiol Neurobiol. 2016; 220:81–87.
Article30. Zelzer E, Mamluk R, Ferrara N, Johnson RS, Schipani E, Olsen BR. VEGFA is necessary for chondrocyte survival during bone development. Development. 2004; 131:2161–2171.
Article31. Zhang C, Li Y, Cornelia R, Swisher S, Kim H. Regulation of VEGF expression by HIF-1α in the femoral head cartilage following ischemia osteonecrosis. Sci Rep. 2012; 2:650.
Article32. Zhang J, Zhu L, Fang J, Ge Z, Li X. LRG1 modulates epithelial-mesenchymal transition and angiogenesis in colorectal cancer via HIF-1α activation. J Exp Clin Cancer Res. 2016; 35:29.
Article33. Zhang LF, Lian LS, Zhao CJ, Li JY, Bao HG, Wu Ch. Expression pattern of HIF1α mRNA in brain, heart and liver tissues of Tibet chicken embryos in hypoxia revealed with quantitative real-time PCR. Animal. 2007; 1:1467–1471.
Article34. Zhang Y, Liu J, Wang S, Luo X, Li Y, Lv Z, Zhu J, Lin J, Ding L, Ye Q. The DEK oncogene activates VEGF expression and promotes tumor angiogenesis and growth in HIF-1α-dependent and -independent manners. Oncotarget. 2016; 7:23740–23756.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hypoxia-inducible factor: role in cell survival in superoxide dismutase overexpressing mice after neonatal hypoxia-ischemia
- Hypoxia-Inducible Factor 1α Regulates the Transforming Growth Factor β1/SMAD Family Member 3 Pathway to Promote Breast Cancer Progression
- Increased Expression of Thymosin β₄ Is Independently Correlated with Hypoxia Inducible Factor-1α (HIF-1α) and Worse Clinical Outcome in Human Colorectal Cancer
- Hypoxia Inducible Factor-1α Directly Induces the Expression of Receptor Activator of Nuclear Factor-κB Ligand in Chondrocytes
- Wheatgrass extract inhibits hypoxia-inducible factor-1-mediated epithelial-mesenchymal transition in A549 cells