Int J Stem Cells.
2017 Nov;10(2):144-153. 10.15283/ijsc17043.
Histological Evaluation of Experimentally Induced Critical Size Defect Skin Wounds Using Exosomal Solution of Mesenchymal Stem Cells Derived Microvesicles
- Affiliations
-
- 1Department of Surgery, Anesthesiology and Radiology, Faculty of Veterinary Medicine, Cairo University, Cairo, Egypt.
- 2Department of Pathology, Animal Health Research Institute, Ministry of Agriculture, Cairo, Egypt.
- 3Department of Surgery, The Military Veterinary Hospital, Cairo, Egypt. omnia.azzam.90@gmail.com
- KMID: 2400868
- DOI: http://doi.org/10.15283/ijsc17043
Abstract
- BACKGROUND AND OBJECTIVES
The present study investigated whether MSCs derived microvesicles (MVs) or (Exosomes) can exert therapeutic effects on an experimental model of cutaneous injury and explored the underlying involving mechanisms.
METHODS AND RESULTS
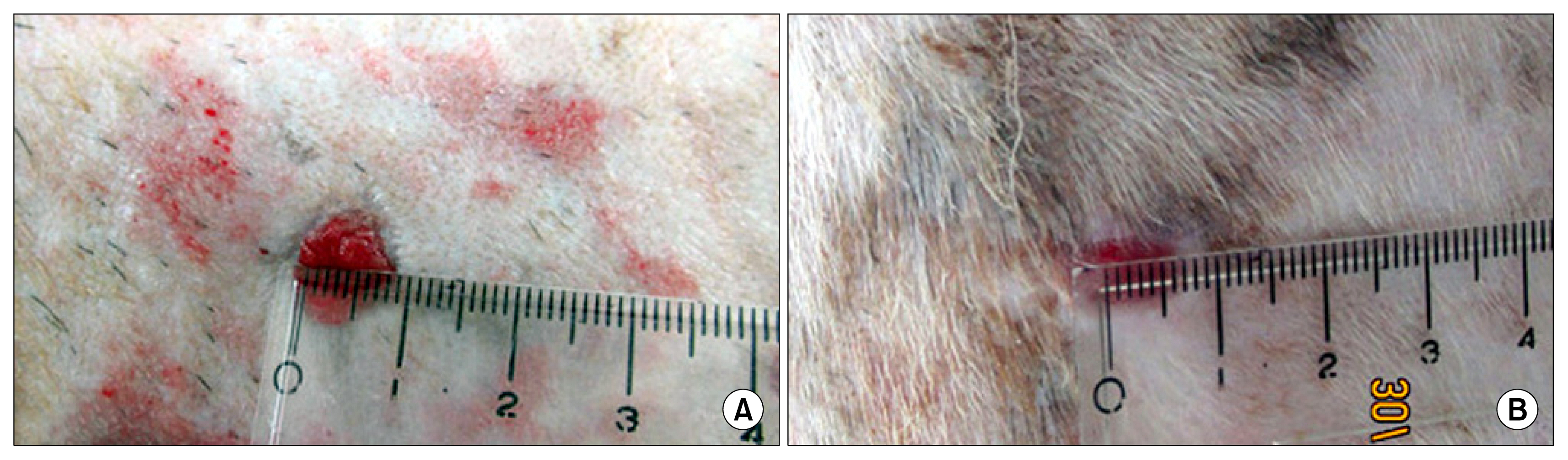
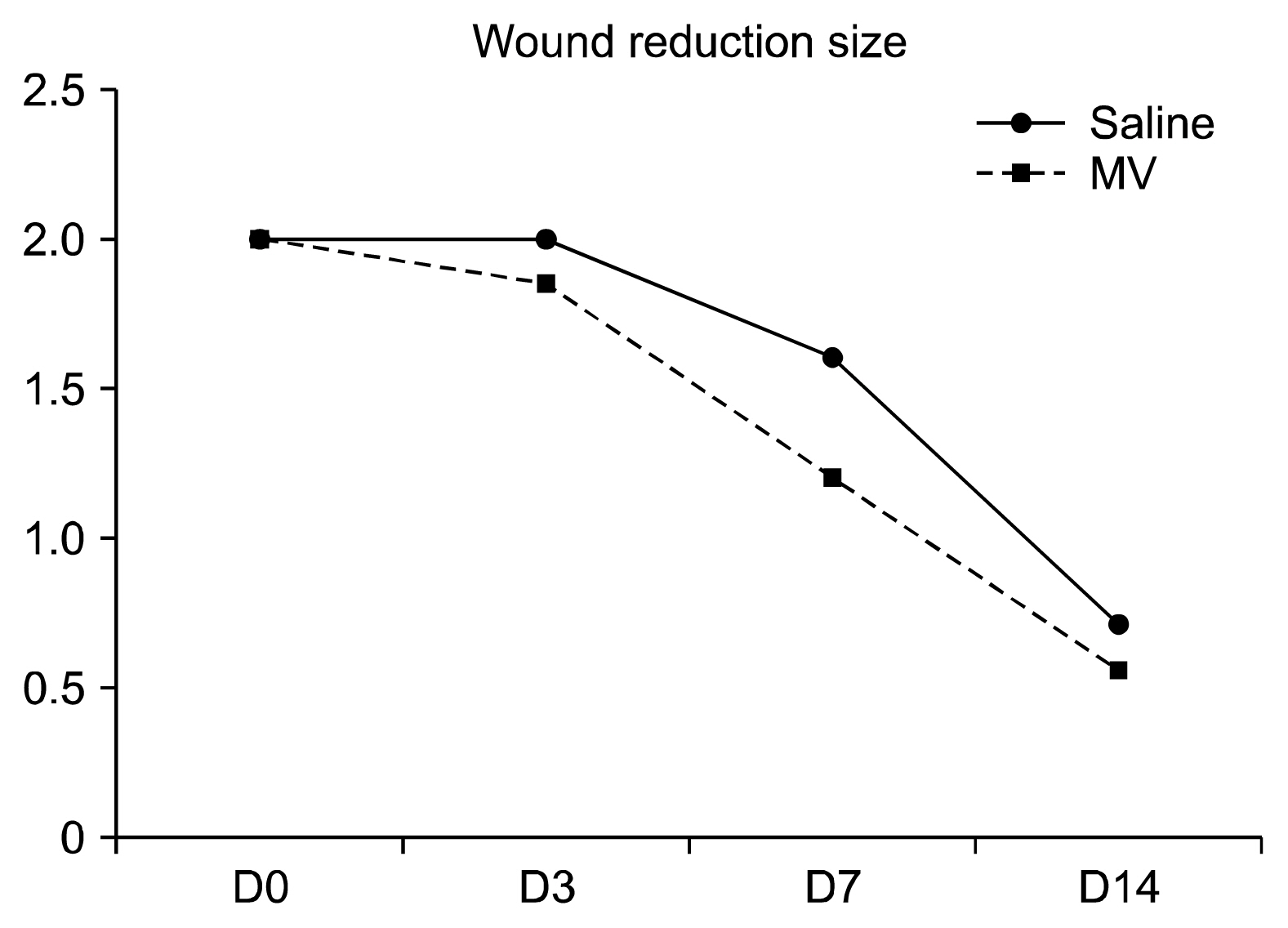
Three bilateral full thickness circular wounds were created on the back of two groups of dogs using 2-cm dermal punch. The wounds were at least 2.5 cm apart. Saline was subcutaneously injected in 4 places around each wound area in group-I (control), whereas an equal volume of exosomal solution of MSCs derived MVs was similarly injected in group-II. The findings demonstrated that MSCs derived MVs had significantly promoted cutaneous wound healing, collagen synthesis, and vascularization at wound sites. The application of the exosomal solution had not only promoted the generation of newly formed vessels, but also have accelerated their development and maturation leading to a faster healing process.
CONCLUSIONS
MSC-Exosomes appeared to be a superior candidate for treating cutaneous wounds than their originator cells, and may represent a promising opportunity to develop a novel cell-free therapy approach that might overcome the obstacles and risks associated with the use of native or engineered stem cells transplantation therapy.
MeSH Terms
Figure
Reference
-
References
1. Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008; 453:314–321. DOI: 10.1038/nature07039. PMID: 18480812.
Article2. Maxson S, Lopez EA, Yoo D, Danilkovitch-Miagkova A, Leroux MA. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med. 2012; 1:142–149. DOI: 10.5966/sctm.2011-0018. PMID: 23197761. PMCID: 3659685.
Article3. Camussi G, Deregibus MC, Cantaluppi V. Role of stem-cell-derived microvesicles in the paracrine action of stem cells. Biochem Soc Trans. 2013; 41:283–287. DOI: 10.1042/BST20120192. PMID: 23356298.
Article4. Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014; 15:4142–4157. DOI: 10.3390/ijms15034142. PMID: 24608926. PMCID: 3975389.
Article5. Fleissner F, Goerzig Y, Haverich A, Thum T. Microvesicles as novel biomarkers and therapeutic targets in transplantation medicine. Am J Transplant. 2012; 12:289–297. DOI: 10.1111/j.1600-6143.2011.03790.x.
Article6. Zhang J, Guan J, Niu X, Hu G, Guo S, Li Q, Xie Z, Zhang C, Wang Y. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015; 13:49. DOI: 10.1186/s12967-015-0417-0. PMID: 25638205. PMCID: 4371881.
Article7. Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther. 2015; 23:812–823. DOI: 10.1038/mt.2015.44. PMID: 25868399. PMCID: 4427881.
Article8. Tögel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007; 292:F1626–F1635. DOI: 10.1152/ajprenal.00339.2006. PMID: 17213465.
Article9. Shabbir A, Zisa D, Suzuki G, Lee T. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: a noninvasive therapeutic regimen. Am J Physiol Heart Circ Physiol. 2009; 296:H1888–H1897. DOI: 10.1152/ajpheart.00186.2009. PMID: 19395555. PMCID: 2716100.
Article10. Shabbir A, Zisa D, Leiker M, Johnston C, Lin H, Lee T. Muscular dystrophy therapy by nonautologous mesenchymal stem cells: muscle regeneration without immunosuppression and inflammation. Transplantation. 2009; 87:1275–1282. DOI: 10.1097/TP.0b013e3181a1719b. PMID: 19424025. PMCID: 2746453.
Article11. Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008; 103:1204–1219. DOI: 10.1161/CIRCRESAHA.108.176826. PMID: 19028920. PMCID: 2667788.
Article12. Simons M, Raposo G. Exosomes--vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009; 21:575–581. DOI: 10.1016/j.ceb.2009.03.007. PMID: 19442504.13. Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004; 94:678–685. DOI: 10.1161/01.RES.0000118601.37875.AC. PMID: 14739163.
Article14. Borena BM, Pawde AM, Amarpal , Aithal HP, Kinjavdekar P, Singh R, Kumar D. Evaluation of autologous bone marrow-derived nucleated cells for healing of full-thickness skin wounds in rabbits. Int Wound J. 2010; 7:249–260. DOI: 10.1111/j.1742-481X.2010.00683.x. PMID: 20492002.
Article15. Luo G, Cheng W, He W, Wang X, Tan J, Fitzgerald M, Li X, Wu J. Promotion of cutaneous wound healing by local application of mesenchymal stem cells derived from human umbilical cord blood. Wound Repair Regen. 2010; 18:506–513. DOI: 10.1111/j.1524-475X.2010.00616.x. PMID: 20840520.
Article16. Badiavas EV, Ford D, Liu P, Kouttab N, Morgan J, Richards A, Maizel A. Long-term bone marrow culture and its clinical potential in chronic wound healing. Wound Repair Regen. 2007; 15:856–865. DOI: 10.1111/j.1524-475X.2007.00305.x. PMID: 18028134.
Article17. Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, Kouttab N, Shrayer D, Carson P. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007; 13:1299–1312. DOI: 10.1089/ten.2006.0278. PMID: 17518741.
Article18. Kim JW, Lee JH, Lyoo YS, Jung DI, Park HM. The effects of topical mesenchymal stem cell transplantation in canine experimental cutaneous wounds. Vet Dermatol. 2013; 24:242–e53. DOI: 10.1111/vde.12011. PMID: 23432413. PMCID: 3618380.
Article19. Fu X, Fang L, Li X, Cheng B, Sheng Z. Enhanced wound-healing quality with bone marrow mesenchymal stem cells autografting after skin injury. Wound Repair Regen. 2006; 14:325–335. DOI: 10.1111/j.1743-6109.2006.00128.x. PMID: 16808812.
Article20. Karayannopoulou M, Tsioli V, Loukopoulos P, Anagnostou TL, Giannakas N, Savvas I, Papazoglou LG, Kaldrymidou E. Evaluation of the effectiveness of an ointment based on Alkannins/Shikonins on second intention wound healing in the dog. Can J Vet Res. 2011; 75:42–48. PMID: 21461194. PMCID: 3003561.21. Adam JA. A simplified model of wound healing (with particular reference to the critical size defect). Mathematical and Computer Modelling. 1999; 30:23–32. DOI: 10.1016/S0895-7177(99)00145-4.
Article22. Ansari MM, Sreekumar TR, Chandra V, Dubey PK, Kumar GS, Amarpal , Sharma GT. Therapeutic potential of canine bone marrow derived mesenchymal stem cells and its conditioned media in diabetic rat wound healing. J Stem Cell Res Ther. 2013; 3:141. DOI: 10.4172/2157-7633.1000141.
Article23. Hiyama A, Mochida J, Iwashina T, Omi H, Watanabe T, Serigano K, Tamura F, Sakai D. Transplantation of mesenchymal stem cells in a canine disc degeneration model. J Orthop Res. 2008; 26:589–600. DOI: 10.1002/jor.20584. PMID: 18203202.
Article24. Zhang Y, Lin HK, Frimberger D, Epstein RB, Kropp BP. Growth of bone marrow stromal cells on small intestinal submucosa: an alternative cell source for tissue engineered bladder. BJU Int. 2005; 96:1120–1125. DOI: 10.1111/j.1464-410X.2005.05741.x. PMID: 16225540.
Article25. Bhattacharya V, McSweeney PA, Shi Q, Bruno B, Ishida A, Nash R, Storb RF, Sauvage LR, Hammond WP, Wu MH. Enhanced endothelialization and microvessel formation in polyester grafts seeded with CD34(+) bone marrow cells. Blood. 2000; 95:581–585. PMID: 10627466.
Article26. Abd-Allah SH, El-Shal AS, Shalaby SM, Abd-Elbary E, Mazen NF, Abdel Kader RR. The role of placenta-derived mesenchymal stem cells in healing of induced full-thickness skin wound in a mouse model. IUBMB Life. 2015; 67:701–709. DOI: 10.1002/iub.1427. PMID: 26315141.
Article27. Basiouny HS, Salama NM, Maadawi ZM, Farag EA. Effect of bone marrow derived mesenchymal stem cells on healing of induced full-thickness skin wounds in albino rat. Int J Stem Cells. 2013; 6:12–25. DOI: 10.15283/ijsc.2013.6.1.12. PMID: 24298370. PMCID: 3840998.
Article28. Tark KC, Hong JW, Kim YS, Hahn SB, Lee WJ, Lew DH. Effects of human cord blood mesenchymal stem cells on cutaneous wound healing in leprdb mice. Ann Plast Surg. 2010; 65:565–572. DOI: 10.1097/SAP.0b013e3181d9aae2. PMID: 20948411.
Article29. Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008; 3:e1886. DOI: 10.1371/journal.pone.0001886. PMID: 18382669. PMCID: 2270908.
Article30. Shabbir A, Cox A, Rodriguez-Menocal L, Salgado M, Van Badiavas E. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev. 2015; 24:1635–1647. DOI: 10.1089/scd.2014.0316. PMID: 25867197. PMCID: 4499790.
Article31. Azari O, Babaei H, Derakhshanfar A, Nematollahi-Mahani SN, Poursahebi R, Moshrefi M. Effects of transplanted mesenchymal stem cells isolated from Wharton’s jelly of caprine umbilical cord on cutaneous wound healing; histopathological evaluation. Vet Res Commun. 2011; 35:211–222. DOI: 10.1007/s11259-011-9464-z. PMID: 21340694.
Article32. Nakagawa H, Akita S, Fukui M, Fujii T, Akino K. Human mesenchymal stem cells successfully improve skin-substitute wound healing. Br J Dermatol. 2005; 153:29–36. DOI: 10.1111/j.1365-2133.2005.06554.x. PMID: 16029323.
Article33. Shi W, Liu L, Li J, Qu L, Pang X, Yu H, Zhang Y, Wang T. Bioactive flavonoids from Flos Sophorae. J Nat Med. 2017; 71:513–522. DOI: 10.1007/s11418-017-1084-7. PMID: 28357634.
Article34. Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999; 341:738–746. DOI: 10.1056/NEJM199909023411006. PMID: 10471461.
Article35. Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997; 276:75–81. DOI: 10.1126/science.276.5309.75. PMID: 9082989.
Article36. Chen L, Xu Y, Zhao J, Zhang Z, Yang R, Xie J, Liu X, Qi S. Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PLoS One. 2014; 9:e96161. DOI: 10.1371/journal.pone.0096161. PMID: 24781370. PMCID: 4004560.
Article37. Hattori N, Mochizuki S, Kishi K, Nakajima T, Takaishi H, D’Armiento J, Okada Y. MMP-13 plays a role in keratinocyte migration, angiogenesis, and contraction in mouse skin wound healing. Am J Pathol. 2009; 175:533–546. DOI: 10.2353/ajpath.2009.081080. PMID: 19590036. PMCID: 2716954.
Article38. Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999; 103:159–165. DOI: 10.1172/JCI5028. PMID: 9916127. PMCID: 407882.
Article39. Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol. 2012; 3:359. DOI: 10.3389/fphys.2012.00359. PMID: 22973239. PMCID: 3434369.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Mesenchymal Stem Cells and Platelet-Derived Growth Factor on the Healing of Radiation Induced Ulcer in Rats
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- MSCs-Derived miR-150-5p-Expressing Exosomes Promote Skin Wound Healing by Activating PI3K/AKT Pathway through PTEN
- Effects of Human Adipose-derived Stem Cells on Cutaneous Wound Healing in Nude Mice
- Effect of Bone Marrow Derived Mesenchymal Stem Cells on Healing of Induced Full-Thickness Skin Wounds in Albino Rat