Immune Netw.
2017 Dec;17(6):424-436. 10.4110/in.2017.17.6.424.
Species Specific Antiviral Activity of Porcine Interferon-α8 (IFNα8)
- Affiliations
-
- 1Laboratory of Cytokine Immunology, Department of Biomedical Science and Technology, Konkuk University, Seoul 05029, Korea. soohyun.kim@konkuk.ac.kr, bearlsy@naver.com
- 2Collage of Veterinary Medicine, Konkuk University, Seoul 05029, Korea.
- 3Research Group of Nutraceuticals for Metabolic Syndrome, Korea Food Research Institute, Jeonbuk 55365, Korea.
- 4Department of Clinical Pathology, Kyungdong University Medical Campus, Wonju-si, Gangwondo 24695, Korea.
- 5Kulf Corporation, Namyangju-si, Gyeonggi-do 12241, Korea.
- 6YbdYbiotech Research Center, Seoul 08589, Korea.
- KMID: 2400628
- DOI: http://doi.org/10.4110/in.2017.17.6.424
Abstract
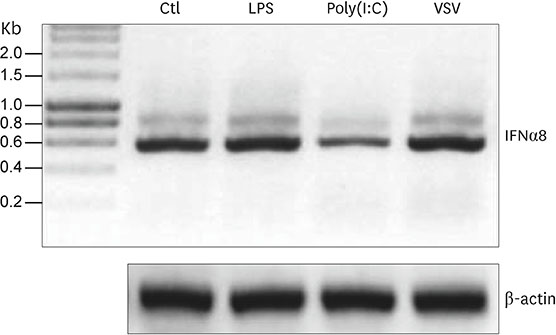
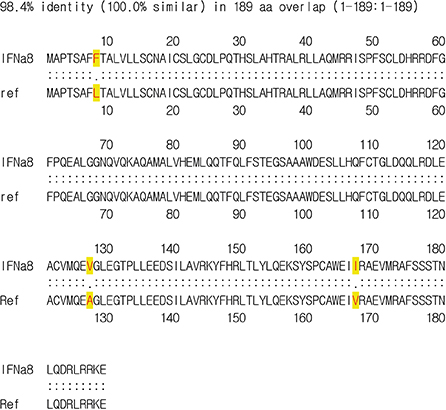
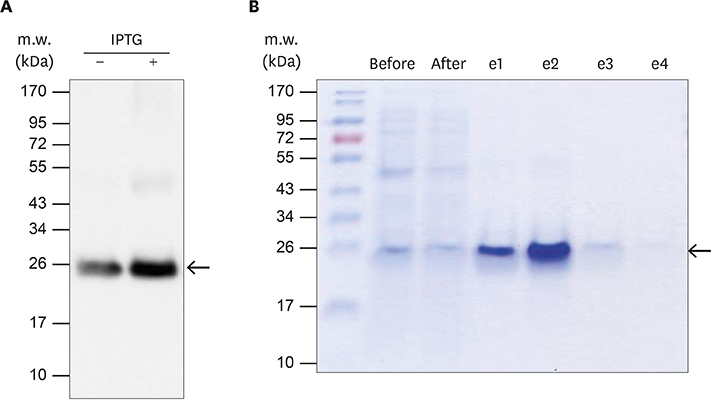
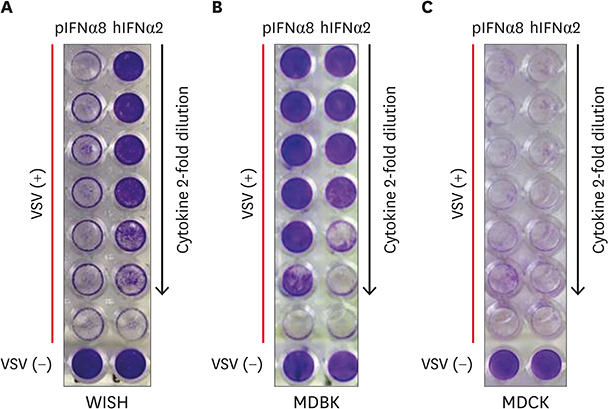
- Interferons (IFNs) have been known as antiviral genes and they are classified by type 1, type 2, and type 3 IFN. The type 1 IFN consists of IFNα, IFNβ, IFNτ, and IFNω whereas the type 2 IFN consists of only IFNγ, which is a key cytokine driving T helper cell type 1 immunity. IFNλ belongs to the type 3 IFN, which is also known as IL-28 and IL-29 possessing antiviral activities. Type 1 IFN is produced by viral infection whereas type 2 IFN is induced by mitogenic or antigenic T-cell stimuli. The IFNτ of bovine was first discovered in an ungulate ruminant recognition hormone. IFNτ belongs to the type 1 IFN with the common feature of type 1 IFN such as antiviral activity. IFNs have been mostly studied for basic research and clinical usages therefore there was no effort to investigate IFNs in industrial animals. Here we cloned porcine IFNα8 from peripheral blood mononuclear cells of Korean domestic pig (Sus scrofa domestica). The newly cloned IFNα8 amino acid sequence from Korean domestic pig shares 98.4% identity with the known porcine IFNα8 in databank. The recombinant porcine IFNα8 showed potent antiviral activity and protected bovine Madin-Darby bovine kidney epithelial (MDBK) cells from the cytopathic effect of vesicular stomatitis virus, but it failed to protect human Wistar Institute Susan Hayflick (WISH) cells and canine Madin-Darby canine kidney epithelial-like (MDCK) cells. The present study demonstrates species specific antiviral activity of porcine IFNα8.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
L1 Recombinant Proteins of HPV Tested for Antibody Forming Using Sera of HPV Quadrivalent Vaccine
Begum Akuzum, Sinae Kim, Tam Thanh Nguyen, Jeawoo Hong, Siyoung Lee, Eunhye Kim, Joohee Kim, Yeook Choi, Hyunjhung Jhun, Youngmin Lee, Hyunwoo Kim, Dong Hyun Sohn, Soohyun Kim
Immune Netw. 2018;18(3):. doi: 10.4110/in.2018.18.e19.
Reference
-
1. Kim SH, Cohen B, Novick D, Rubinstein M. Mammalian type I interferon receptors consists of two subunits: IFNaR1 and IFNaR2. Gene. 1997; 196:279–286.
Article2. Novick D, Cohen B, Rubinstein M. The human interferon alpha/beta receptor: characterization and molecular cloning. Cell. 1994; 77:391–400.
Article3. Novick D, Nabioullin RR, Ragsdale W, McKenna S, Weiser W, Garone L, Burkins C, Kim SH, Rubinstein M, Tepper MA, et al. The neutralization of type I IFN biologic actions by anti-IFNAR-2 monoclonal antibodies is not entirely due to inhibition of Jak-Stat tyrosine phosphorylation. J Interferon Cytokine Res. 2000; 20:971–982.
Article4. Lee S, Choi DK, Kwak A, Kim S, Nguyen TT, Gil G, Kim E, Yoo KH, Kim IA, Lee Y, et al. IL-32-induced inflammatory cytokines are selectively suppressed by α1-antitrypsin in mouse bone marrow cells. Immune Netw. 2017; 17:116–120.
Article5. Kwak A, Lee Y, Kim H, Kim S. Intracellular interleukin (IL)-1 family cytokine processing enzyme. Arch Pharm Res. 2016; 39:1556–1564.
Article6. Kim S. Interleukin-32 in inflammatory autoimmune diseases. Immune Netw. 2014; 14:123–127.
Article7. Kim B, Lee Y, Kim E, Kwak A, Ryoo S, Bae SH, Azam T, Kim S, Dinarello CA. The interleukin-1α precursor is biologically active and is likely a key alarmin in the IL-1 family of cytokines. Front Immunol. 2013; 4:391.
Article8. Bach EA, Aguet M, Schreiber RD. The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol. 1997; 15:563–591.9. Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, Bendelac A. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999; 163:4647–4650.10. Young HA. Regulation of interferon-gamma gene expression. J Interferon Cytokine Res. 1996; 16:563–568.11. Frucht DM, Fukao T, Bogdan C, Schindler H, O'Shea JJ, Koyasu S. IFN-gamma production by antigen-presenting cells: mechanisms emerge. Trends Immunol. 2001; 22:556–560.12. Gessani S, Belardelli F. IFN-gamma expression in macrophages and its possible biological significance. Cytokine Growth Factor Rev. 1998; 9:117–123.13. Sen GC. Viruses and interferons. Annu Rev Microbiol. 2001; 55:255–281.
Article14. Ank N, West H, Bartholdy C, Eriksson K, Thomsen AR, Paludan SR. Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J Virol. 2006; 80:4501–4509.
Article15. Gray CA, Abbey CA, Beremand PD, Choi Y, Farmer JL, Adelson DL, Thomas TL, Bazer FW, Spencer TE. Identification of endometrial genes regulated by early pregnancy, progesterone, and interferon tau in the ovine uterus. Biol Reprod. 2006; 74:383–394.
Article16. Binelli M, Subramaniam P, Diaz T, Johnson GA, Hansen TR, Badinga L, Thatcher WW. Bovine interferon-tau stimulates the Janus kinase-signal transducer and activator of transcription pathway in bovine endometrial epithelial cells. Biol Reprod. 2001; 64:654–665.
Article17. Roberts RM. Interferon-tau, a type 1 interferon involved in maternal recognition of pregnancy. Cytokine Growth Factor Rev. 2007; 18:403–408.
Article18. Roberts RM, Liu L, Alexenko A. New and atypical families of type I interferons in mammals: comparative functions, structures, and evolutionary relationships. Prog Nucleic Acid Res Mol Biol. 1997; 56:287–325.19. Uzé G, Lutfalla G, Gresser I. Genetic transfer of a functional human interferon alpha receptor into mouse cells: cloning and expression of its cDNA. Cell. 1990; 60:225–234.
Article20. Bazer FW, Spencer TE, Ott TL. Interferon tau: a novel pregnancy recognition signal. Am J Reprod Immunol. 1997; 37:412–420.
Article21. Chen Y, Green JA, Antoniou E, Ealy AD, Mathialagan N, Walker AM, Avalle MP, Rosenfeld CS, Hearne LB, Roberts RM. Effect of interferon-tau administration on endometrium of nonpregnant ewes: a comparison with pregnant ewes. Endocrinology. 2006; 147:2127–2137.
Article22. Roberts RM, Cross JC, Leaman DW. Interferons as hormones of pregnancy. Endocr Rev. 1992; 13:432–452.
Article23. Spencer TE, Becker WC, George P, Mirando MA, Ogle TF, Bazer FW. Ovine interferon-tau regulates expression of endometrial receptors for estrogen and oxytocin but not progesterone. Biol Reprod. 1995; 53:732–745.
Article24. Wolfsdorf KE, Diaz T, Schmitt EJ, Thatcher MJ, Drost M, Thatcher WW. The dominant follicle exerts an interovarian inhibition on FSH-induced follicular development. Theriogenology. 1997; 48:435–447.
Article25. Robinson RS, Mann GE, Lamming GE, Wathes DC. The effect of pregnancy on the expression of uterine oxytocin, oestrogen and progesterone receptors during early pregnancy in the cow. J Endocrinol. 1999; 160:21–33.
Article26. Pontzer CH, Bazer FW, Johnson HM. Antiproliferative activity of a pregnancy recognition hormone, ovine trophoblast protein-1. Cancer Res. 1991; 51:5304–5307.27. Pontzer CH, Torres BA, Vallet JL, Bazer FW, Johnson HM. Antiviral activity of the pregnancy recognition hormone ovine trophoblast protein-1. Biochem Biophys Res Commun. 1988; 152:801–807.
Article28. Skopets B, Li J, Thatcher WW, Roberts RM, Hansen PJ. Inhibition of lymphocyte proliferation by bovine trophoblast protein-1 (type I trophoblast interferon) and bovine interferon-alpha I1. Vet Immunol Immunopathol. 1992; 34:81–96.
Article29. Soos JM, Subramaniam PS, Hobeika AC, Schiffenbauer J, Johnson HM. The IFN pregnancy recognition hormone IFN-tau blocks both development and superantigen reactivation of experimental allergic encephalomyelitis without associated toxicity. J Immunol. 1995; 155:2747–2753.30. Kim H, Gil G, Lee S, Kwak A, Jo S, Kim E, Nguyen TT, Kim S, Jhun H, Kim S, et al. Cytokine-like activity of liver type fatty acid binding protein (L-FABP) inducing inflammatory cytokine interleukin-6. Immune Netw. 2016; 16:296–304.
Article31. Lee S, Kim E, Jhun H, Hong J, Kwak A, Jo S, Bae S, Lee J, Kim B, Lee J, et al. Proinsulin shares a motif with interleukin-1α (IL-1α) and induces inflammatory cytokine via interleukin-1 receptor 1. J Biol Chem. 2016; 291:14620–14627.
Article32. Aida Y, Pabst MJ. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J Immunol Methods. 1990; 132:191–195.
Article33. Kang D, Ryoo S, Chung B, Lee J, Park S, Han J, Jeong S, Rho G, Hong J, Bae S, et al. Amino acid differences in interferon-tau (IFN-τ) of Bos taurus Coreanae and Holstein. Cytokine. 2012; 59:273–279.
Article34. Uzé G, Schreiber G, Piehler J, Pellegrini S. The receptor of the type I interferon family. Curr Top Microbiol Immunol. 2007; 316:71–95.
Article35. Kerr IM, Brown RE. pppA2'p5'A2'p5'A: an inhibitor of protein synthesis synthesized with an enzyme fraction from interferon-treated cells. Proc Natl Acad Sci USA. 1978; 75:256–260.
Article36. Rebouillat D, Hovanessian AG. The human 2′,5′-oligoadenylate synthetase family: interferon-induced proteins with unique enzymatic properties. J Interferon Cytokine Res. 1999; 19:295–308.
Article37. Rebouillat D, Hovnanian A, Marié I, Hovanessian AG. The 100-kDa 2′,5′-oligoadenylate synthetase catalyzing preferentially the synthesis of dimeric pppA2'p5'A molecules is composed of three homologous domains. J Biol Chem. 1999; 274:1557–1565.
Article38. Ghosh A, Desai SY, Sarkar SN, Ramaraj P, Ghosh SK, Bandyopadhyay S, Sen GC. Effects of mutating specific residues present near the amino terminus of 2′-5′-oligoadenylate synthetase. J Biol Chem. 1997; 272:15452–15458.
Article39. Ghosh A, Sarkar SN, Guo W, Bandyopadhyay S, Sen GC. Enzymatic activity of 2′-5′-oligoadenylate synthetase is impaired by specific mutations that affect oligomerization of the protein. J Biol Chem. 1997; 272:33220–33226.
Article40. Sarkar SN, Bandyopadhyay S, Ghosh A, Sen GC. Enzymatic characteristics of recombinant medium isozyme of 2′-5′ oligoadenylate synthetase. J Biol Chem. 1999; 274:1848–1855.
Article41. Sarkar SN, Ghosh A, Wang HW, Sung SS, Sen GC. The nature of the catalytic domain of 2′-5′-oligoadenylate synthetases. J Biol Chem. 1999; 274:25535–25542.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Mechanisms of Interferon - alpha and Interferon - gamma Induced Antiviral Activity in Peritoneal Macrophages
- Direct Antiviral Mechanisms of Interferon-Gamma
- Evaluation of Antiviral Activity of
Zanthoxylum Species Against Picornaviruses - Activation of Porcine Oocytes Following Intracytoplasmic Injection of Various Sperm Components and foreign species spermatozoa
- Antiviral Activity of Corylus heterophylla Fisch Against Porcine Epidemic Diarrhea Virus Infection