Ann Rehabil Med.
2017 Dec;41(6):905-914. 10.5535/arm.2017.41.6.905.
Neural Correlates of Motor Recovery Measured by SPECT at Six Months After Basal Ganglia Stroke
- Affiliations
-
- 1Department of Rehabilitation Medicine, Wonkwang University School of Medicine and Hospital, Iksan, Korea. helmaine@naver.com
- 2Department of Nuclear Medicine, Wonkwang University School of Medicine and Hospital, Iksan, Korea.
- KMID: 2400274
- DOI: http://doi.org/10.5535/arm.2017.41.6.905
Abstract
OBJECTIVE
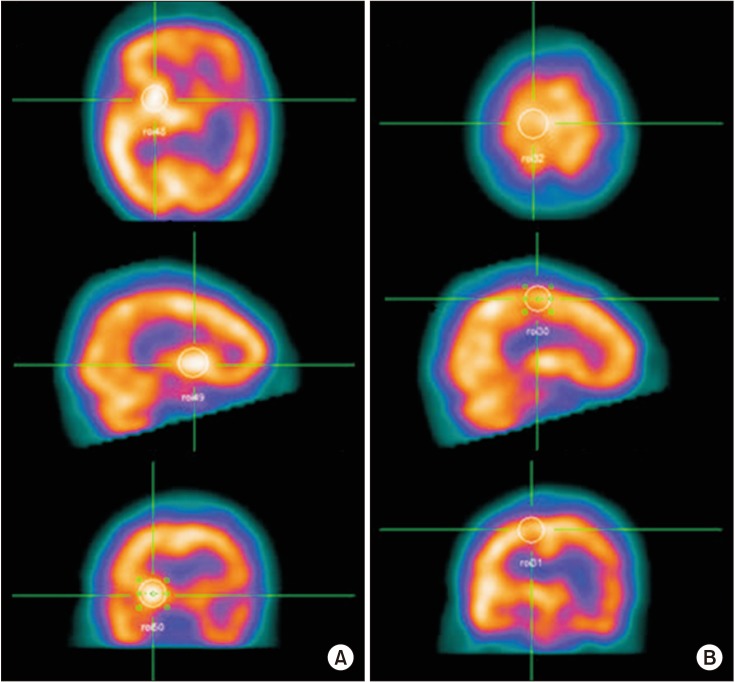
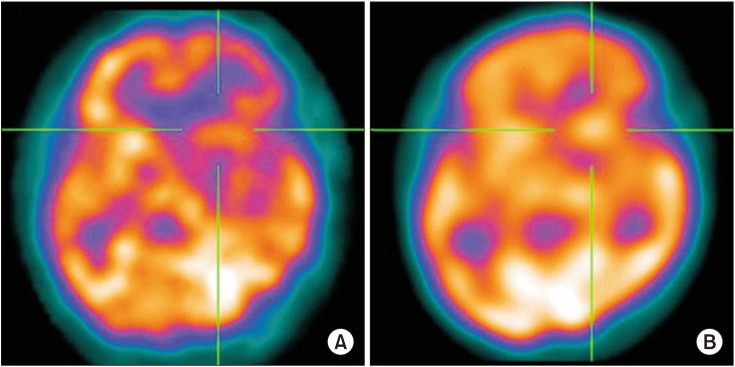
To investigate neural correlates associated with recovery of motor function over 6 months in patients with basal ganglia (BG) stroke using acetazolamide (ACZ) stress brain-perfusion single-photon emission computed tomography (SPECT).
METHODS
Medical records of 22 patients presenting first-ever BG stroke were retrospectively reviewed. Regional cerebral blood flow (CBF) and cerebrovascular reserve (CVR) were measured for 9 regions in each cerebral hemisphere (primary motor cortex, supplementary motor area, premotor cortex, prefrontal cortex, temporal lobe, parietal lobe, occipital lobe, BG, and thalamus). The Fugl-Meyer Assessment (FMA) motor score was used to assess motor function.
RESULTS
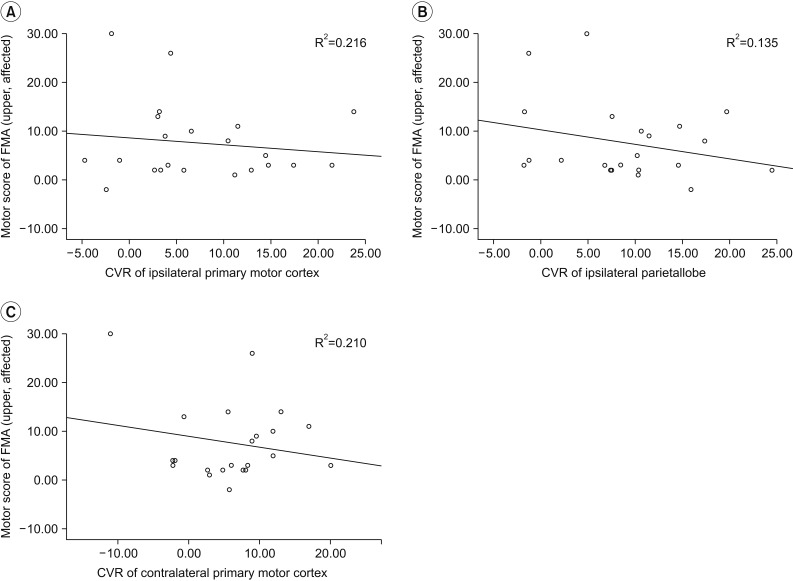
After ACZ injection, CBF of all regions of interest (ROIs) increased compared with baseline. Baseline CBF of all ROIs was not significantly correlated with changes in FMA upper or lower motor score. However, multivariate analysis revealed CVR was significantly associated with change in FMA upper score in the ipsilateral primary motor cortex (R2=0.216, p=0.017), the ipsilateral parietal lobe (R2=0.135, p=0.029), and the contralateral primary motor cortex (R2=0.210, p=0.041).
CONCLUSION
CVR in the bilateral primary motor cortex and ipsilateral parietal lobe was associated with restoration of upper motor function 6 months after BG stroke. SPECT is a readily available imaging modality useful in studying brain residual function in patients with BG stroke.
Keyword
MeSH Terms
-
Acetazolamide
Basal Ganglia*
Brain
Cerebrovascular Circulation
Cerebrum
Humans
Medical Records
Motor Cortex
Multivariate Analysis
Occipital Lobe
Parietal Lobe
Prefrontal Cortex
Recovery of Function
Retrospective Studies
Stroke*
Temporal Lobe
Tomography, Emission-Computed
Tomography, Emission-Computed, Single-Photon*
Acetazolamide
Figure
Reference
-
1. Stinear C. Prediction of recovery of motor function after stroke. Lancet Neurol. 2010; 9:1228–1232. PMID: 21035399.
Article2. Groenewegen HJ. The basal ganglia and motor control. Neural Plast. 2003; 10:107–120. PMID: 14640312.
Article3. Hikosaka O, Nakamura K, Sakai K, Nakahara H. Central mechanisms of motor skill learning. Curr Opin Neurobiol. 2002; 12:217–222. PMID: 12015240.
Article4. Grefkes C, Fink GR. Connectivity-based approaches in stroke and recovery of function. Lancet Neurol. 2014; 13:206–216. PMID: 24457190.
Article5. Carey LM, Abbott DF, Egan GF, O'Keefe GJ, Jackson GD, Bernhardt J, et al. Evolution of brain activation with good and poor motor recovery after stroke. Neurorehabil Neural Repair. 2006; 20:24–41. PMID: 16467276.
Article6. Calautti C, Leroy F, Guincestre JY, Baron JC. Dynamics of motor network overactivation after striatocapsular stroke: a longitudinal PET study using a fixed-performance paradigm. Stroke. 2001; 32:2534–2542. PMID: 11692013.
Article7. Ward NS, Newton JM, Swayne OB, Lee L, Thompson AJ, Greenwood RJ, et al. Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain. 2006; 129(Pt 3):809–819. PMID: 16421171.
Article8. Lindenberg R, Renga V, Zhu LL, Betzler F, Alsop D, Schlaug G. Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology. 2010; 74:280–287. PMID: 20101033.
Article9. Vagal AS, Leach JL, Fernandez-Ulloa M, Zuccarello M. The acetazolamide challenge: techniques and applications in the evaluation of chronic cerebral ischemia. AJNR Am J Neuroradiol. 2009; 30:876–884. PMID: 19246526.
Article10. Nuutinen J, Liu Y, Laakso MP, Karonen JO, Vanninen EJ, Kuikka JT, et al. Perfusion differences on SPECT and PWI in patients with acute ischemic stroke. Neuroradiology. 2009; 51:687–695. PMID: 19626319.
Article11. Kononen M, Kuikka JT, Husso-Saastamoinen M, Vanninen E, Vanninen R, Soimakallio S, et al. Increased perfusion in motor areas after constraint-induced movement therapy in chronic stroke: a single-photon emission computerized tomography study. J Cereb Blood Flow Metab. 2005; 25:1668–1674. PMID: 15931162.12. Takekawa T, Kakuda W, Uchiyama M, Ikegaya M, Abo M. Brain perfusion and upper limb motor function: a pilot study on the correlation between evolution of asymmetry in cerebral blood flow and improvement in Fugl-Meyer Assessment score after rTMS in chronic post-stroke patients. J Neuroradiol. 2014; 41:177–183. PMID: 23886875.
Article13. Park SA, Park HI, Kim D, Yang CY, Zhang LQ. The prediction of gross motor outcome using cerebrovascular reserve measured by acetazolamide-challenged SPECT. NeuroRehabilitation. 2012; 30:359–367. PMID: 22672952.
Article14. Hattori N, Yonekura Y, Tanaka F, Fujita T, Wang J, Ishizu K, et al. One-day protocol for cerebral perfusion reserve with acetazolamide. J Nucl Med. 1996; 37:2057–2061. PMID: 8970534.15. Haggard P. Human volition: towards a neuroscience of will. Nat Rev Neurosci. 2008; 9:934–946. PMID: 19020512.
Article16. Seitz RJ, Azari NP, Knorr U, Binkofski F, Herzog H, Freund HJ. The role of diaschisis in stroke recovery. Stroke. 1999; 30:1844–1850. PMID: 10471434.
Article17. Andaluz N, Choutka O, Vagal A, Strunk R, Zuccarello M. Patient selection for revascularization procedures in adult Moyamoya disease based on dynamic perfusion computerized tomography with acetazolamide challenge (PCTA). Neurosurg Rev. 2010; 33:225–232. PMID: 20140634.
Article18. Iida H, Nakagawara J, Hayashida K, Fukushima K, Watabe H, Koshino K, et al. Multicenter evaluation of a standardized protocol for rest and acetazolamide cerebral blood flow assessment using a quantitative SPECT reconstruction program and split-dose 123I-iodoamphetamine. J Nucl Med. 2010; 51:1624–1631. PMID: 20847163.
Article19. Yonas H, Darby JM, Marks EC, Durham SR, Maxwell C. CBF measured by Xe-CT: approach to analysis and normal values. J Cereb Blood Flow Metab. 1991; 11:716–725. PMID: 1908474.
Article20. Kapucu OL, Nobili F, Varrone A, Booij J, Vander Borght T, Nagren K, et al. EANM procedure guideline for brain perfusion SPECT using 99mTc-labelled radiopharmaceuticals, version 2. Eur J Nucl Med Mol Imaging. 2009; 36:2093–2102. PMID: 19838703.
Article21. Cramer SC, Parrish TB, Levy RM, Stebbins GT, Ruland SD, Lowry DW, et al. Predicting functional gains in a stroke trial. Stroke. 2007; 38:2108–2114. PMID: 17540966.
Article22. Hultborn H, Nielsen JB. Spinal control of locomotion: from cat to man. Acta Physiol (Oxf). 2007; 189:111–121. PMID: 17250563.23. Luft AR, Smith GV, Forrester L, Whitall J, Macko RF, Hauser TK, et al. Comparing brain activation associated with isolated upper and lower limb movement across corresponding joints. Hum Brain Mapp. 2002; 17:131–140. PMID: 12353246.
Article24. Rehme AK, Eickhoff SB, Wang LE, Fink GR, Grefkes C. Dynamic causal modeling of cortical activity from the acute to the chronic stage after stroke. Neuroimage. 2011; 55:1147–1158. PMID: 21238594.
Article25. Favre I, Zeffiro TA, Detante O, Krainik A, Hommel M, Jaillard A. Upper limb recovery after stroke is associated with ipsilesional primary motor cortical activity: a meta-analysis. Stroke. 2014; 45:1077–1083. PMID: 24525953.26. Park CH, Chang WH, Ohn SH, Kim ST, Bang OY, Pascual-Leone A, et al. Longitudinal changes of resting-state functional connectivity during motor recovery after stroke. Stroke. 2011; 42:1357–1362. PMID: 21441147.
Article27. Rehme AK, Fink GR, von Cramon DY, Grefkes C. The role of the contralesional motor cortex for motor recovery in the early days after stroke assessed with longitudinal FMRI. Cereb Cortex. 2011; 21:756–768. PMID: 20801897.
Article28. Nowak DA, Grefkes C, Dafotakis M, Eickhoff S, Kust J, Karbe H, et al. Effects of low-frequency repetitive transcranial magnetic stimulation of the contralesional primary motor cortex on movement kinematics and neural activity in subcortical stroke. Arch Neurol. 2008; 65:741–747. PMID: 18541794.
Article29. Schulz R, Buchholz A, Frey BM, Bonstrup M, Cheng B, Thomalla G, et al. Enhanced effective connectivity between primary motor cortex and intraparietal sulcus in well-recovered stroke patients. Stroke. 2016; 47:482–489. PMID: 26742802.
Article30. Schulz R, Koch P, Zimerman M, Wessel M, Bonstrup M, Thomalla G, et al. Parietofrontal motor pathways and their association with motor function after stroke. Brain. 2015; 138(Pt 7):1949–1960. PMID: 25935722.
Article31. O'Shea J, Sebastian C, Boorman ED, Johansen-Berg H, Rushworth MF. Functional specificity of human premotor-motor cortical interactions during action selection. Eur J Neurosci. 2007; 26:2085–2095. PMID: 17868374.32. O'Shea J, Johansen-Berg H, Trief D, Gobel S, Rushworth MF. Functionally specific reorganization in human premotor cortex. Neuron. 2007; 54:479–490. PMID: 17481399.33. Park CH, Chang WH, Lee M, Kwon GH, Kim L, Kim ST, et al. Predicting the performance of motor imagery in stroke patients: multivariate pattern analysis of functional MRI data. Neurorehabil Neural Repair. 2015; 29:247–254. PMID: 25055835.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Motor Recovery in Relation to the Site of Subcortical Stroke Lesions
- Hypersexuality and Obsessive-Compulsive Behaviors in a Stroke Patient with the Left Mesial Frontal Cortex and Both Basal Ganglia Lesion
- Clinical and Neuropsychological Characteristics of Patients with Stroke of the Basal Ganglia
- Movement Disorders Following Cerebrovascular Lesion in the Basal Ganglia Circuit
- Crossed Cerebellar Diaschisis: Risk Factors and Correlation to Functional Recovery in Intracerebral Hemorrhage