Pediatr Gastroenterol Hepatol Nutr.
2017 Dec;20(4):236-243. 10.5223/pghn.2017.20.4.236.
Short-Term Outcome of Infliximab Therapy in Pediatric Crohn's Disease: A Single-Center Experience
- Affiliations
-
- 1Department of Pediatrics, Asan Medical Center Children's Hospital, University of Ulsan College of Medicine, Seoul, Korea. seakhee.oh@amc.seoul.kr
- KMID: 2398905
- DOI: http://doi.org/10.5223/pghn.2017.20.4.236
Abstract
- PURPOSE
Studies on the efficacy of infliximab (IFX) in a large population of pediatric patients with Crohn's disease (CD) are limited, and prognostic factors are not well-known. The aim of this study was to evaluate outcomes of IFX in pediatric patients with CD and to identify factors associated with poor prognosis.
METHODS
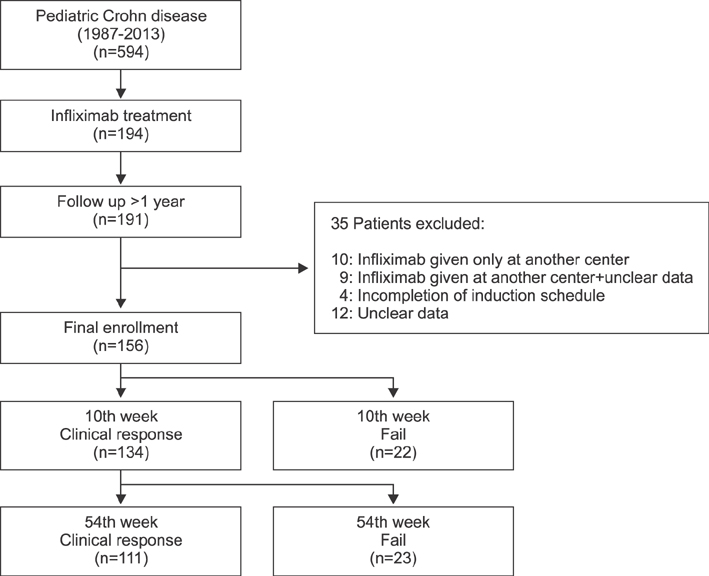
We retrospectively analyzed medical data of 594 pediatric patients with CD between 1987 and 2013 in a tertiary center. Of these, 156 children treated with IFX were enrolled and were followed up for at least a year with intact data. Outcomes of induction and maintenance, classified as failure or clinical response, were evaluated on the tenth and 54th week of IFX therapy.
RESULTS
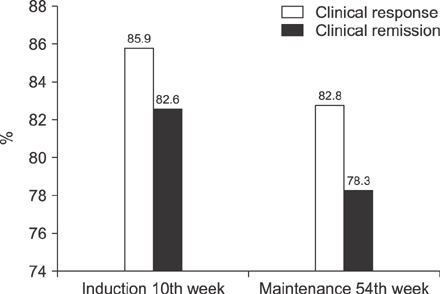
We treated 156 pediatric patients with CD with IFX, and the median duration of IFX therapy was 47 months. For IFX induction therapy, 134 (85.9%) patients experienced clinical response on the 10th week. Among the 134 patients who showed response to induction, 111 (82.8%) patients maintained the clinical response on the 54th week. In multivariate analysis, low hematocrit (p=0.046) at the time of IFX initiation was associated with the failure of IFX induction. For IFX maintenance therapy, longer duration from the initial diagnosis to IFX therapy (p=0.017) was associated with maintenance failure on the 54th week.
CONCLUSION
We have shown the acceptable outcomes of IFX in a large cohort of pediatric CD patients in Korea. Hematocrit and early introduction of IFX may be prognostic factors for the outcomes of IFX.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Mucosal Immunity Related to FOXP3+ Regulatory T Cells, Th17 Cells and Cytokines in Pediatric Inflammatory Bowel Disease
Jinhee Cho, Sorina Kim, Da Hee Yang, Juyeon Lee, Kyeong Won Park, Junyong Go, Chang-Lim Hyun, Youngheun Jee, Ki Soo Kang
J Korean Med Sci. 2018;33(52):. doi: 10.3346/jkms.2018.33.e336.
Reference
-
1. Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012; 142:46–54.e42. quiz e30.
Article2. Adamiak T, Walkiewicz-Jedrzejczak D, Fish D, Brown C, Tung J, Khan K, et al. Incidence, clinical characteristics, and natural history of pediatric IBD in Wisconsin: a population-based epidemiological study. Inflamm Bowel Dis. 2013; 19:1218–1223.
Article3. Kim BJ, Song SM, Kim KM, Lee YJ, Rhee KW, Jang JY, et al. Characteristics and trends in the incidence of inflammatory bowel disease in Korean children: a single-center experience. Dig Dis Sci. 2010; 55:1989–1995.
Article4. Grossi V, Hyams JS. The safety of treatment options for pediatric Crohn's disease. Expert Opin Drug Saf. 2016; 15:1383–1390.
Article5. Ruemmele FM, Veres G, Kolho KL, Griffiths A, Levine A, Escher JC, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. J Crohns Colitis. 2014; 8:1179–1207.
Article6. Park JJ, Yang SK, Ye BD, Kim JW, Park DI, Yoon H, et al. Second Korean guidelines for the management of Crohn's disease. Intest Res. 2017; 15:38–67.
Article7. Hosoi K, Ohtsuka Y, Fujii T, Kudo T, Matsunaga N, Tomomasa T, et al. Treatment with infliximab for pediatric Crohn's disease: nationwide survey of Japan. J Gastroenterol Hepatol. 2017; 32:114–119.
Article8. Lee YM, Kang B, Lee Y, Kim MJ, Choe YH. Infliximab “top-down” strategy is superior to “step-up” in maintaining long-term remission in the treatment of pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2015; 60:737–743.
Article9. Luo Y, Yu J, Zhao H, Peng K, Lou J, Ma M, et al. Efficacy of infliximab in treatment of pediatric Crohn's disease in China. Zhonghua Er Ke Za Zhi. 2014; 52:688–692.10. Hyams J, Crandall W, Kugathasan S, Griffiths A, Olson A, Johanns J, et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn's disease in children. Gastroenterology. 2007; 132:863–873. quiz 1165-6.
Article11. Walters TD, Kim MO, Denson LA, Griffiths AM, Dubinsky M, Markowitz J, et al. Increased effectiveness of early therapy with anti-tumor necrosis factor-α vs an immunomodulator in children with Crohn's disease. Gastroenterology. 2014; 146:383–391.
Article12. deBruyn JC, Jacobson K, El-Matary W, Carroll M, Wine E, Wrobel I, et al. Long-term outcomes of infliximab use for pediatric Crohn's disease: a canadian multicenter clinical practice experience. J Pediatr Gastroenterol Nutr. 2017; DOI: 10.1097/MPG.0000000000001672. [Epub ahead of print].13. Hyams JS, Lerer T, Griffiths A, Pfefferkorn M, Kugathasan S, Evans J, et al. Long-term outcome of maintenance infliximab therapy in children with Crohn's disease. Inflamm Bowel Dis. 2009; 15:816–822.
Article14. Vahabnezhad E, Rabizadeh S, Dubinsky MC. A 10-year, single tertiary care center experience on the durability of infliximab in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2014; 20:606–613.
Article15. IBD Working Group of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition. Inflammatory bowel disease in children and adolescents: recommendations for diagnosis--the Porto criteria. J Pediatr Gastroenterol Nutr. 2005; 41:1–7.16. Levine A, Griffiths A, Markowitz J, Wilson DC, Turner D, Russell RK, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011; 17:1314–1321.
Article17. Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn's disease activity index. National cooperative Crohn's disease study. Gastroenterology. 1976; 70:439–444.18. Hyams JS, Ferry GD, Mandel FS, Gryboski JD, Kibort PM, Kirschner BS, et al. Development and validation of a pediatric Crohn's disease activity index. J Pediatr Gastroenterol Nutr. 1991; 12:439–447.
Article19. Hyams J, Markowitz J, Otley A, Rosh J, Mack D, Bousvaros A, et al. Evaluation of the pediatric crohn disease activity index: a prospective multicenter experience. J Pediatr Gastroenterol Nutr. 2005; 41:416–421.
Article20. Grossi V, Lerer T, Griffiths A, LeLeiko N, Cabrera J, Otley A, et al. Concomitant use of immunomodulators affects the durability of infliximab therapy in children with Crohn's disease. Clin Gastroenterol Hepatol. 2015; 13:1748–1756.
Article21. Church PC, Guan J, Walters TD, Frost K, Assa A, Muise AM, et al. Infliximab maintains durable response and facilitates catch-up growth in luminal pediatric Crohn's disease. Inflamm Bowel Dis. 2014; 20:1177–1186.
Article22. Crombé V, Salleron J, Savoye G, Dupas JL, Vernier-Massouille G, Lerebours E, et al. Long-term outcome of treatment with infliximab in pediatric-onset Crohn’s disease: a population-based study. Inflamm Bowel Dis. 2011; 17:2144–2152.
Article23. Ruffolo C, Scarpa M, Bassi N. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010; 363:1086–1087.
Article24. Koutroubakis IE, Ramos-Rivers C, Regueiro M, Koutroumpakis E, Click B, Schoen RE, et al. Persistent or recurrent anemia is associated with severe and disabling inflammatory bowel disease. Clin Gastroenterol Hepatol. 2015; 13:1760–1766.
Article25. Rieder F, Paul G, Schnoy E, Schleder S, Wolf A, Kamm F, et al. Hemoglobin and hematocrit levels in the prediction of complicated Crohn's disease behavior--a cohort study. PLoS One. 2014; 9:e104706.26. Koutroubakis IE, Ramos-Rivers C, Regueiro M, Koutroumpakis E, Click B, Schwartz M, et al. The influence of anti-tumor necrosis factor agents on hemoglobin levels of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2015; 21:1587–1593.
Article27. Bergamaschi G, Di Sabatino A, Albertini R, Ardizzone S, Biancheri P, Bonetti E, et al. Prevalence and pathogenesis of anemia in inflammatory bowel disease. Influence of anti-tumor necrosis factor-alpha treatment. Haematologica. 2010; 95:199–205.
Article28. Nahon S, Lahmek P, Paupard T, Lesgourgues B, Chaussade S, Peyrin-Biroulet L, et al. Diagnostic delay is associated with a greater risk of early surgery in a French cohort of Crohn's disease patients. Dig Dis Sci. 2016; 61:3278–3284.
Article29. Ma C, Beilman CL, Huang VW, Fedorak DK, Kroeker KI, Dieleman LA, et al. Anti-TNF therapy within 2 years of Crohn's disease diagnosis improves patient outcomes: a retrospective cohort study. Inflamm Bowel Dis. 2016; 22:870–879.
Article30. Oh EH, Oh K, Han M, Seo H, Chang K, Lee SH, et al. Early anti-TNF/immunomodulator therapy is associated with better long-term clinical outcomes in Asian patients with Crohn's disease with poor prognostic factors. PLoS One. 2017; 12:e0177479.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Trends of Infliximab Treatment for Crohn's Disease
- Factors Affecting Surgical Treatment With Infliximab Therapy in Perianal Fistula With Crohn Disease
- Adalimumab Treatment in Pediatric-Onset Crohn's Disease Patients after Infliximab Failure: A Single Center Study
- Efficacy of Early Infliximab Treatment for Pediatric Crohn's Disease: A Three-year Follow-up
- Change in the treatment strategy for pediatric Crohn's disease