Diabetes Metab J.
2017 Dec;41(6):466-473. 10.4093/dmj.2017.41.6.466.
The Phospholipid Linoleoylglycerophosphocholine as a Biomarker of Directly Measured Insulin Resistance
- Affiliations
-
- 1Department of Medicine, Universidad de los Andes School of Medicine, Bogotá, Colombia. cmendivi@uniandes.edu.co
- 2Molecular Epidemiology of Endocrine Diseases Group, Universidad Militar Nueva Granada, School of Medicine, Bogotá, Colombia.
- 3Department of Chemistry, Universidad de los Andes, Bogotá, Colombia.
- 4Section of Endocrinology, Fundación Santa Fe de Bogotá, Bogotá, Colombia.
- KMID: 2398087
- DOI: http://doi.org/10.4093/dmj.2017.41.6.466
Abstract
- BACKGROUND
Plasma concentrations of some lysophospholipids correlate with metabolic alterations in humans, but their potential as biomarkers of insulin resistance (IR) is insufficiently known. We aimed to explore the association between plasma linoleoylglycerophosphocholine (LGPC) and objective measures of IR in adults with different metabolic profiles.
METHODS
We studied 62 men and women, ages 30 to 69 years, (29% normal weight, 59% overweight, 12% obese). Participants underwent a 5-point oral glucose tolerance test (5p-OGTT) from which we calculated multiple indices of IR and insulin secretion. Fifteen participants additionally underwent a hyperinsulinemic-euglycemic clamp for estimation of insulin-stimulated glucose disposal. Plasma LGPC was determined using high performance liquid chromatography/time-of-flight mass spectrometry. Plasma LGPC was compared across quartiles defined by the IR indices.
RESULTS
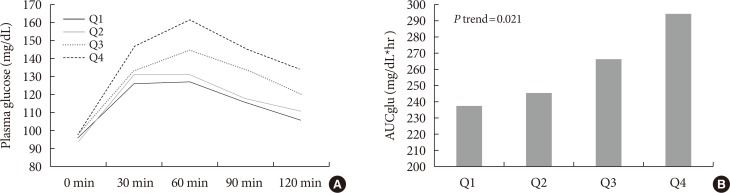
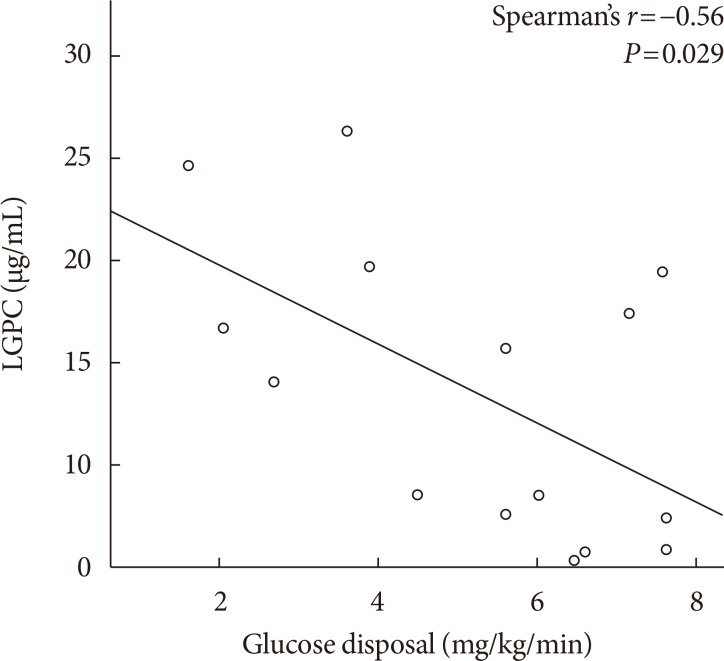
Mean LGPC was 15.4±7.6 ng/mL in women and 14.1±7.3 ng/mL in men. LGPC did not correlate with body mass in-dex, percent body fat, waist circumference, blood pressure, glycosylated hemoglobin, log-triglycerides, or high density lipoprotein cholesterol. Plasma LGPC concentrations was not systematically associated with any of the studied 5p-OGTT-derived IR indices. However, LGPC exhibited a significant negative correlation with glucose disposal in the clamp (Spearman r=−0.56, P=0.029). Despite not being diabetic, participants with higher plasma LGPC exhibited significantly higher post-challenge plasma glucose excursions in the 5p-OGTT (P trend=0.021 for the increase in glucose area under the curve across quartiles of plasma LGPC).
CONCLUSION
In our sample of Latino adults without known diabetes, LGPC showed potential as a biomarker of IR and impaired glucose metabolism.
Keyword
MeSH Terms
-
Adipose Tissue
Adiposity
Adult
Biomarkers
Blood Glucose
Blood Pressure
Cholesterol, HDL
Female
Glucose
Glucose Tolerance Test
Hemoglobin A, Glycosylated
Hispanic Americans
Humans
Insulin Resistance*
Insulin*
Lysophospholipids
Male
Mass Spectrometry
Metabolism
Metabolome
Obesity
Overweight
Plasma
Waist Circumference
Biomarkers
Cholesterol, HDL
Glucose
Insulin
Lysophospholipids
Figure
Reference
-
1. Kullmann S, Heni M, Hallschmid M, Fritsche A, Preissl H, Haring HU. Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol Rev. 2016; 96:1169–1209. PMID: 27489306.
Article2. Heise T, Zijlstra E, Nosek L, Heckermann S, Plum-Morschel L, Forst T. Euglycaemic glucose clamp: what it can and cannot do, and how to do it. Diabetes Obes Metab. 2016; 18:962–972. PMID: 27324560.
Article3. Meikle PJ, Summers SA. Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat Rev Endocrinol. 2017; 13:79–91. PMID: 27767036.
Article4. Borkman M, Storlien LH, Pan DA, Jenkins AB, Chisholm DJ, Campbell LV. The relation between insulin sensitivity and the fatty-acid composition of skeletal-muscle phospholipids. N Engl J Med. 1993; 328:238–244. PMID: 8418404.
Article5. Sandra A, Fyler DJ. The effect of membrane phospholipid modification on insulin action in adipocytes from normal and streptozotocin-induced diabetic rats. Horm Metab Res. 1982; 14:638–641. PMID: 6759360.
Article6. Cobb J, Eckhart A, Motsinger-Reif A, Carr B, Groop L, Ferrannini E. α-Hydroxybutyric acid is a selective metabolite biomarker of impaired glucose tolerance. Diabetes Care. 2016; 39:988–995. PMID: 27208342.
Article7. Floegel A, Stefan N, Yu Z, Muhlenbruch K, Drogan D, Joost HG, Fritsche A, Haring HU, Hrabe de Angelis M, Peters A, Roden M, Prehn C, Wang-Sattler R, Illig T, Schulze MB, Adamski J, Boeing H, Pischon T. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes. 2013; 62:639–648. PMID: 23043162.
Article8. Wang-Sattler R, Yu Z, Herder C, Messias AC, Floegel A, He Y, Heim K, Campillos M, Holzapfel C, Thorand B, Grallert H, Xu T, Bader E, Huth C, Mittelstrass K, Doring A, Meisinger C, Gieger C, Prehn C, Roemisch-Margl W, Carstensen M, Xie L, Yamanaka-Okumura H, Xing G, Ceglarek U, Thiery J, Giani G, Lickert H, Lin X, Li Y, Boeing H, Joost HG, de Angelis MH, Rathmann W, Suhre K, Prokisch H, Peters A, Meitinger T, Roden M, Wichmann HE, Pischon T, Adamski J, Illig T. Novel biomarkers for pre-diabetes identified by metabolomics. Mol Syst Biol. 2012; 8:615. PMID: 23010998.
Article9. Ferrannini E, Natali A, Camastra S, Nannipieri M, Mari A, Adam KP, Milburn MV, Kastenmuller G, Adamski J, Tuomi T, Lyssenko V, Groop L, Gall WE. Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes. 2013; 62:1730–1737. PMID: 23160532.
Article10. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979; 237:E214–E223. PMID: 382871.
Article11. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28:412–419. PMID: 3899825.12. Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000; 85:2402–2410. PMID: 10902785.
Article13. Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990; 300:230–235. PMID: 2106931.
Article14. Gutt M, Davis CL, Spitzer SB, Llabre MM, Kumar M, Czarnecki EM, Schneiderman N, Skyler JS, Marks JB. Validation of the insulin sensitivity index (ISI(0,120)): comparison with other measures. Diabetes Res Clin Pract. 2000; 47:177–184. PMID: 10741566.
Article15. Sluiter WJ, Erkelens DW, Reitsma WD, Doorenbos H. Glucose tolerance and insulin release, a mathematical approach I. Assay of the beta-cell response after oral glucose loading. Diabetes. 1976; 25:241–244. PMID: 773721.
Article16. Barber MN, Risis S, Yang C, Meikle PJ, Staples M, Febbraio MA, Bruce CR. Plasma lysophosphatidylcholine levels are reduced in obesity and type 2 diabetes. PLoS One. 2012; 7:e41456. PMID: 22848500.
Article17. Liang H, Hussey SE, Sanchez-Avila A, Tantiwong P, Musi N. Effect of lipopolysaccharide on inflammation and insulin action in human muscle. PLoS One. 2013; 8:e63983. PMID: 23704966.
Article18. Moreno-Navarrete JM, Ortega F, Serino M, Luche E, Waget A, Pardo G, Salvador J, Ricart W, Fruhbeck G, Burcelin R, Fernandez-Real JM. Circulating lipopolysaccharide-binding protein (LBP) as a marker of obesity-related insulin resistance. Int J Obes (Lond). 2012; 36:1442–1449. PMID: 22184060.
Article19. Rauschert S, Uhl O, Koletzko B, Kirchberg F, Mori TA, Huang RC, Beilin LJ, Hellmuth C, Oddy WH. Lipidomics reveals associations of phospholipids with obesity and insulin resistance in young adults. J Clin Endocrinol Metab. 2016; 101:871–879. PMID: 26709969.
Article20. Del Bas JM, Caimari A, Rodriguez-Naranjo MI, Childs CE, Paras Chavez C, West AL, Miles EA, Arola L, Calder PC. Impairment of lysophospholipid metabolism in obesity: altered plasma profile and desensitization to the modulatory properties of n-3 polyunsaturated fatty acids in a randomized controlled trial. Am J Clin Nutr. 2016; 104:266–279. PMID: 27305954.
Article21. Jonas A. Lecithin cholesterol acyltransferase. Biochim Biophys Acta. 2000; 1529:245–256. PMID: 11111093.
Article22. Borggreve SE, De Vries R, Dullaart RP. Alterations in high-density lipoprotein metabolism and reverse cholesterol transport in insulin resistance and type 2 diabetes mellitus: role of lipolytic enzymes, lecithin:cholesterol acyltransferase and lipid transfer proteins. Eur J Clin Invest. 2003; 33:1051–1069. PMID: 14636288.
Article23. Cole LK, Vance JE, Vance DE. Phosphatidylcholine biosynthesis and lipoprotein metabolism. Biochim Biophys Acta. 2012; 1821:754–761. PMID: 21979151.
Article24. Wentworth JM, Naselli G, Ngui K, Smyth GK, Liu R, O'Brien PE, Bruce C, Weir J, Cinel M, Meikle PJ, Harrison LC. GM3 ganglioside and phosphatidylethanolamine-containing lipids are adipose tissue markers of insulin resistance in obese women. Int J Obes (Lond). 2016; 40:706–713. PMID: 26499445.
Article25. Arendt BM, Ma DW, Simons B, Noureldin SA, Therapondos G, Guindi M, Sherman M, Allard JP. Nonalcoholic fatty liver disease is associated with lower hepatic and erythrocyte ratios of phosphatidylcholine to phosphatidylethanolamine. Appl Physiol Nutr Metab. 2013; 38:334–340. PMID: 23537027.
Article