Asia Pac Allergy.
2012 Oct;2(4):256-263. 10.5415/apallergy.2012.2.4.256.
Interferon-alpha inhibits airway eosinophila and hyperresponsiveness in an animal asthma model
- Affiliations
-
- 1Department of Pulmonary Medicine and Clinical Immunology, Dokkyo Medical University, 880 Kita-kobayashi, Mibu, Tochigi 321-0293, Japan. sugiyama@dokkyomed.ac.jp
- KMID: 2397442
- DOI: http://doi.org/10.5415/apallergy.2012.2.4.256
Abstract
- BACKGROUND
Asthma is characterized by a chronic inflammatory process involving high numbers of inflammatory cells and mediators which have multiple inflammatory effects on the airway. Interferon (IFN)-alpha, which is used widely for treating chronic hepatitis C, is reported to have an effect on patients with Churg-Strauss syndrome. Therefore, it may also be suitable for patients with severe asthma.
OBJECTIVE
We studied the effect of IFN-alpha on airway eosinophilia in a guinea pig model of asthma and the expression of adhesion molecules on human eosinophils and vascular endothelial cells.
METHODS
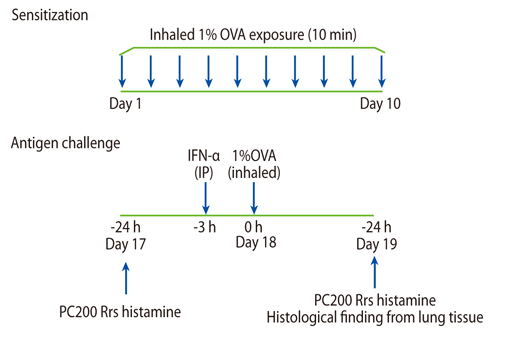
After antigen challenge, airway hyperresponsiveness and airway eosinophilia were measured in a guinea pig asthma model with or without airway IFN-alpha administration. Expression of adhesion molecules on eosinophils and cultured human umbilical vein endothelial cells (HUVECs) was also evaluated with or without IFN-alpha.
RESULTS
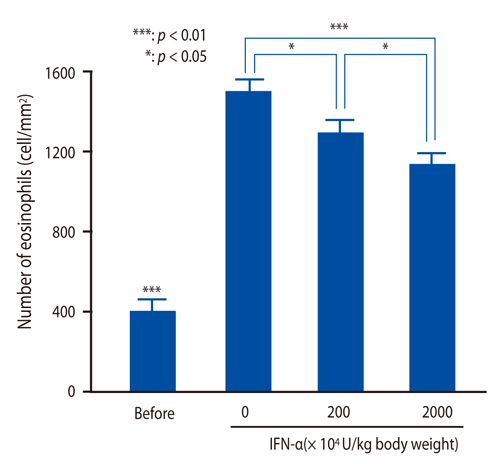
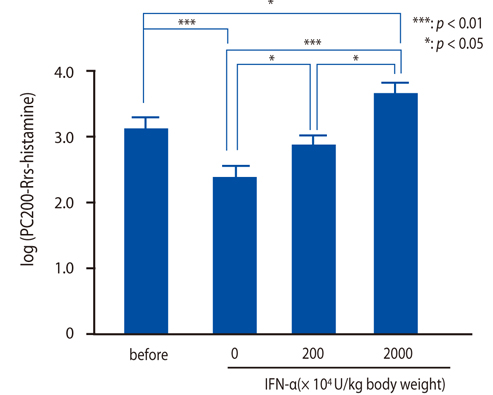
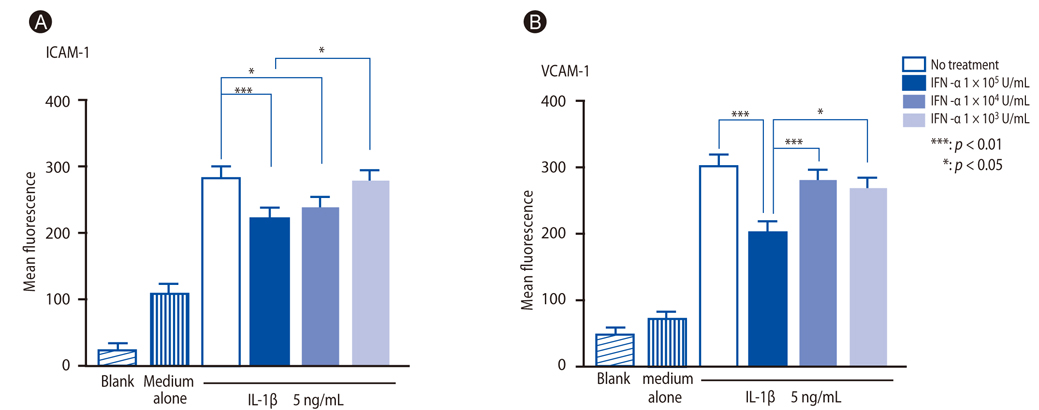
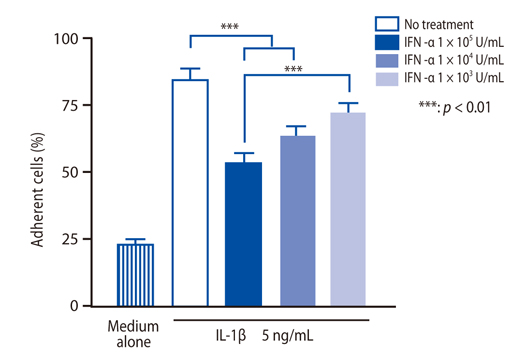
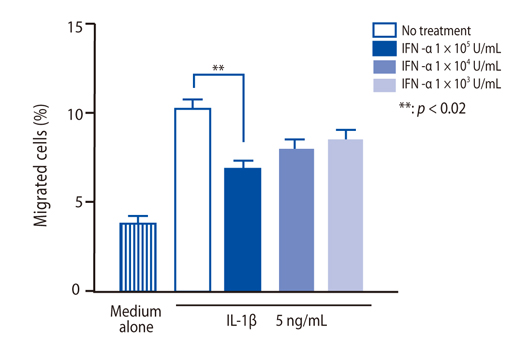
IFN-alpha inhibited eosinophil recruitment into the tracheal wall and improved airway hyperresponsiveness in sensitized guinea pigs. IFN-alpha also significantly suppressed IL-1 beta-induced intercellular adhesion molecule-1 (ICAM-1) expression on HUVECs. However, IFN-alpha did not suppress platelet-activating factor-induced macrophage antigen-1 expression on human eosinophils. IFN-alpha significantly inhibited eosinophil adhesion to IL-1 beta-induced HUVECs and migration through IL-1 beta induced HUVECs.
CONCLUSION
The findings suggest that the modulation of ICAM-1 in lung with pre-existing inflammation following treatment with IFN-alpha may be a novel and selective treatment for control of chronic airway inflammation and hyperresponsiveness associated with asthma.
Keyword
MeSH Terms
-
Animals*
Asthma*
Churg-Strauss Syndrome
Endothelial Cells
Eosinophilia
Eosinophils
Guinea Pigs
Hepatitis C, Chronic
Human Umbilical Vein Endothelial Cells
Humans
Inflammation
Intercellular Adhesion Molecule-1
Interferon-alpha*
Interferons
Interleukin-1
Interleukin-1beta
Lung
Macrophages
Intercellular Adhesion Molecule-1
Interferon-alpha
Interferons
Interleukin-1
Interleukin-1beta
Figure
Cited by 2 articles
-
The Interplay between Host Immunity and Respiratory Viral Infection in Asthma Exacerbation
Ferdaus Mohd Altaf Hossain, Jin Young Choi, Erdenebileg Uyangaa, Seong Ok Park, Seong Kug Eo
Immune Netw. 2019;19(5):. doi: 10.4110/in.2019.19.e31.Implementation of guidelines, allergy programs, and the October issue
Yoon-Seok Chang
Asia Pac Allergy. 2012;2(4):231-232. doi: 10.5415/apallergy.2012.2.4.231.
Reference
-
1. Barnes PJ. New concepts in the pathogenesis of bronchial hyperresponsiveness and asthma. J Allergy Clin Immunol. 1989. 83:1013–1026.
Article2. Bousquet J, Chanez P, Lacoste JY, Barnéon G, Ghavanian N, Enander I, Venge P, Ahlstedt S, Simony-Lafontaine J, Godard P, Michel FB. Eosinophilic inflammation in asthma. N Engl J Med. 1990. 323:1033–1039.
Article3. Lopez AF, Sanderson CJ, Gamble JR, Campbell HD, Young IG, Vadas MA. Recombinant human interleukin 5 is a selective activator of human eosinophil function. J Exp Med. 1988. 167:219–224.
Article4. Wang JM, Rambaldi A, Biondi A, Chen ZG, Sanderson CJ, Mantovani A. Recombinant human interleukin 5 is a selective eosinophil chemoattractant. Eur J Immunol. 1989. 19:701–705.
Article5. Fukuda T, Nakajima H, Fukushima Y, Akutsu I, Numao T, Majima K, Motojima S, Sato Y, Takatsu K, Makino S. Detection of interleukin-5 messenger RNA and interleukin-5 protein in bronchial biopsies from asthma by nonradioactive in situ hybridization and immunohistochemistry. J Allergy Clin Immunol. 1994. 94:584–593.
Article6. Adachi T, Motojima S, Hirata A, Fukuda T, Makino S. Eosinophil viability-enhancing activity in sputum from patients with bronchial asthma, Contributions of interleukin-5 and granulocyte/macrophage colony-stimulating factor. Am J Respir Crit Care Med. 1995. 151:618–623.
Article7. Sur S, Kita H, Gleich GJ, Chenier TC, Hunt LW. Eosinophil recruitment is associated with IL-5, but not with RANTES, twenty-four hours after allergen challenge. J Allergy Clin Immunol. 1996. 97:1272–1278.
Article8. Sanmugalingham D, De Vries E, Gauntlett R, Symon FA, Bradding P, Wardlaw AJ. Interleukin-5 enhances eosinophil adhesion to bronchial epithelial cells. Clin Exp Allergy. 2000. 30:255–263.
Article9. Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001. 358:958–965.
Article10. Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL Jr, Häussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002. 347:975–982.
Article11. Poordad F, McCone J Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N, DiNubile MJ, Sniukiene V, Brass CA, Albrecht JK, Bronowicki JP. SPRINT-2 Investigators. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011. 364:1195–1206.
Article12. Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R, George J, Rizzetto M, Shouval D, Sola R, Terg RA, Yoshida EM, Adda N, Bengtsson L, Sankoh AJ, Kieffer TL, George S, Kauffman RS, Zeuzem S. ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011. 364:2405–2416.
Article13. Shibuya H, Hirohata S. Differential effects of IFN-alpha on the expression of various TH2 cytokines in human CD4+ T cells. J Allergy Clin Immunol. 2005. 116:205–212.14. Tilg H, Kaser A. Type I interferons and their therapeutic role in Th2-regulated inflammatory disorders. Expert Opin Biol Ther. 2004. 4:469–481.
Article15. Chung KF, Barnes PJ. Cytokines in asthma. Thorax. 1999. 54:825–857.
Article16. Schandené L, Del Prete GF, Cogan E, Stordeur P, Crusiaux A, Kennes B, Romagnani S, Goldman M. Recombinant interferon-alpha selectively inhibits the production of interleukin-5 by human CD4+ T cells. J Clin Invest. 1996. 97:309–315.
Article17. Nakajima H, Nakao A, Watanabe Y, Yoshida S, Iwamoto I. IFN-alpha inhibits antigen-induced eosinophil and CD4+ T cell recruitment into tissue. J Immunol. 1994. 153:1264–1270.18. Tatsis E, Schnabel A, Gross WL. Interferon-alpha treatment of four patients with the Churg-Strauss syndrome. Ann Intern Med. 1998. 129:370–374.19. Termeer CC, Simon JC, Schöpf E. Low-dose interferon alfa-2b for the treatment of Churg-Strauss syndrome with prominent skin involvement. Arch Dermatol. 2001. 137:136–138.20. Hellmich B, Gross WL. Recent progress in the pharmacotherapy of Churg-Strauss syndrome. Expert Opin Pharmacother. 2004. 5:25–35.
Article21. Cheng G, Ueda T, Sugiyama K, Toda M, Fukuda T. Compositional and functional changes of pulmonary surfactant in a guinea-pig model of chronic asthma. Respir Med. 2001. 95:180–186.
Article22. Mead J. Control of respiratory frequency. J Appl Physiol. 1960. 15:325–336.
Article23. Toda M, Motojima S, Fukuda T, Makino S. Effect of an herbal preparation, Saiboku-to (TJ-96), on antigen-induced airway hyperresponsiveness and eosinophil infiltration in actively sensitized guinea pigs. off. Ann N Y Acad Sci. 1993. 685:561–571.24. Tenor H, Hatzelmann A, Church MK, Schudt C, Shute JK. Effects of theophylline and rolipram on leukotriene C4 (LTC4) synthesis and chemotaxis of human eosinophils from normal and atopic subjects. Br J Pharmacol. 1996. 118:1727–1735.
Article25. Ebisawa M, Bochner BS, Georas SN, Schleimer RP. Eosinophil transendothelial migration induced by cytokines. I. Role of endothelial and eosinophil adhesion molecules in IL-1 beta-induced transendothelial migration. J Immunol. 1992. 149:4021–4028.26. Gratzl S, Palca A, Schmitz M, Simon HU. Treatment with IFN-alpha in corticosteroid-unresponsive asthma. J Allergy Clin Immunol. 2000. 105:1035–1036.27. Simon HU, Seelbach H, Ehmann R, Schmitz M. Clinical and immunological effects of low-dose IFN-alpha treatment in patients with corticosteroid-resistant asthma. Allergy. 2003. 58:1250–1255.28. Kroegel C, Bergmann N, Foerster M, Workalemahu G, Machnik A, Mock B, Reissig A. Interferon-alphacon-1 treatment of three patients with severe glucocorticoid-dependent asthma. Effect on disease control and systemic glucocorticosteroid dose. Respiration. 2006. 73:566–570.29. Bini EJ, Weinshel EH. Severe exacerbation of asthma: a new side effect of interferon-alpha in patients with asthma and chronic hepatitis C. Mayo Clin Proc. 1999. 74:367–370.30. Shirai T, Honjo Y, Takashima M, Takayanagi S, Chida K, Nakamura H. Effect of interferon-α on pulmonary function and airway responsiveness in patients with chronic hepatitis C. Allergol Int. 2001. 50:331–335.
Article31. Kanazawa H, Mamoto T, Hirata K, Yoshikawa J. Interferon therapy induces the improvement of lung function by inhaled corticosteroid therapy in asthmatic patients with chronic hepatitis C virus infection: a preliminary study. Chest. 2003. 123:600–603.32. Capra F, De Maria E, Lunardi C, Marchiori L, Mezzelani P, Beri R, Gabrielli GB. Serum level of soluble intercellular adhesion molecule 1 in patients with chronic liver disease related to hepatitis C virus: A prognostic marker for responses to interferon treatment. J Infect Dis. 2000. 181:425–431.
Article33. Dejica D, Grigorescu M, Dejica V, Radu C, Neculoiu D. Serum levels of soluble intercellular-1 and vascular cell-1 adhesion molecules in chronic hepatitis C and the influence of interferon-alpha + ribavirin therapy. Rom J Gastroenterol. 2002. 11:277–283.34. Pober JS, Gimbrone MA Jr, Lapierre LA, Mendrick DL, Fiers W, Rothlein R, Springer TA. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986. 137:1893–1896.35. Schindler R, Ghezzi P, Dinarello CA. Interferons as inhibitors of interleukin 1 induced interleukin 1 synthesis. Lymphokine Res. 1989. 8:275–280.36. Håkansson A, Gustafsson B, Krysander L, Hjelmqvist B, Rettrup B, Håkansson L. Expression of ICAM-1 during IFN-alpha-based treatment of metastatic malignant melanoma: relation to tumor-infiltrating mononuclear cells and regressive tumor changes. J Interferon Cytokine Res. 1999. 19:171–177.37. Schaefer M, Horn M, Schmidt F, Schmid-Wendtner MH, Volkenandt M, Ackenheil M, Mueller N, Schwarz MJ. Correlation between sICAM-1 and depressive symptoms during adjuvant treatment of melanoma with interferon-alpha. Brain Behav Immun. 2004. 18:555–562.38. Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996. 17:138–146.
Article39. Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science. 1996. 272:50–53.
Article40. Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003. 3:133–146.
Article41. Bochner BS, Luscinskas FW, Gimbrone MA Jr, Newman W, Sterbinsky SA, Derse-Anthony CP, Klunk D, Schleimer RP. Adhesion of human basophils, eosinophils, and neutrophils to interleukin 1-activated human vascular endothelial cells: contributions of endothelial cell adhesion molecules. J Exp Med. 1991. 173:1553–1557.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Interferon-alpha inhibits airway eosinophila and hyperresponsiveness in an animal asthma model
- Effects of Korean Red Ginseng Extracts on Airway Hyperresponsiveness and Inflammation in a Murine Asthma Model
- Effects of CpG-oligodeoxynucleotides in Chronic Inflammation and Remodeling of Airway in a Murine Model of Bronchial Asthma
- Respiratory Syncutial Virus Infection Induced Airway Hyperresponsiveness
- Effect of DHEA on airway hyperresponsiveness and inflammation in murine model of asthma