Asia Pac Allergy.
2016 Apr;6(2):77-89. 10.5415/apallergy.2016.6.2.77.
T-cell-mediated drug hypersensitivity: immune mechanisms and their clinical relevance
- Affiliations
-
- 1Department of Clinical Immunology and Allergy, Royal North Shore Hospital, St Leonards, NSW 2065, Australia. James.Yun@health.nsw.gov.au
- 2Department of Clinical Immunology, Royal Prince Alfred Hospital, Camperdown, NSW 2050, Australia.
- 3ADR-AC GmbH, Bern 3008, Switzerland.
- KMID: 2396975
- DOI: http://doi.org/10.5415/apallergy.2016.6.2.77
Abstract
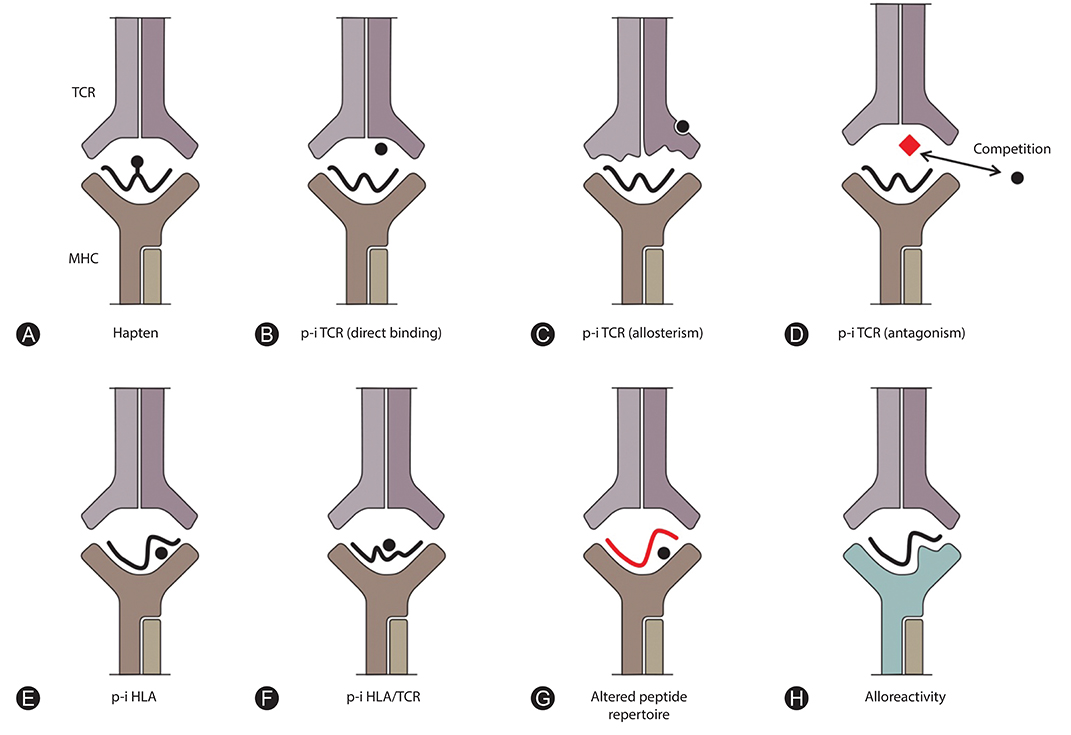
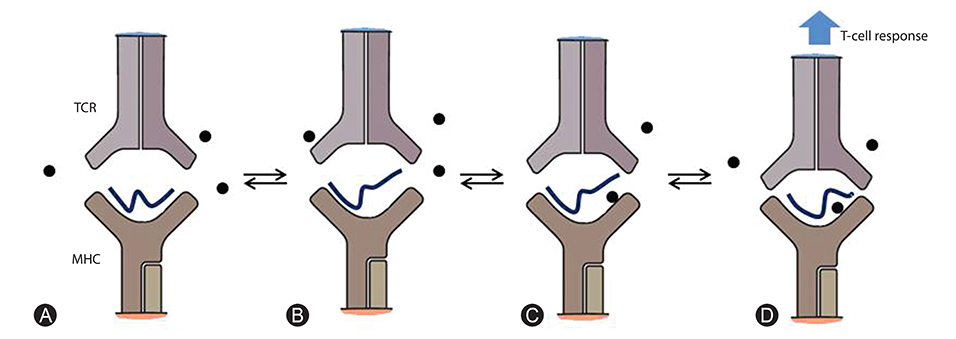
- T-cell-mediated drug hypersensitivity represents a significant proportion of immune mediated drug hypersensitivity reactions. In the recent years, there has been an increase in understanding the immune mechanisms behind T-cell-mediated drug hypersensitivity. According to hapten mechanism, drug specific T-cell response is stimulated by drug-protein conjugate presented on major histocompatibility complex (MHC) as it is presented as a new antigenic determinant. On the other hand, p-i concept suggests that a drug can stimulate T cells via noncovalent direct interaction with T-cell receptor and/or peptide-MHC. The drug binding site is quite variable and this leads to several different mechanisms within p-i concept. Altered peptide repertoire can be regarded as an 'atypical' subset of p-i concept since the mode of the drug binding and the binding site are essentially identical to p-i concept. However, the intracellular binding of abacavir to HLA-B*57:01 additionally results in alteration in peptide repertoire. Furthermore the T-cell response to altered peptide repertoire model is only shown for abacavir and HLA-B*57:01 and therefore it may not be generalised to other drug hypersensitivity. Danger hypothesis has been postulated to play an important role in drug hypersensitivity by providing signal 2 but its experimental data is lacking at this point in time. Furthermore, the recently described allo-immune response suggests that danger signal may be unnecessary. Finally, in view of these new understanding, the classification and the definition of type B adverse drug reaction should be revised.
MeSH Terms
Figure
Cited by 2 articles
-
The era of allergy
Yoon-Seok Chang
Asia Pac Allergy. 2016;6(2):75-76. doi: 10.5415/apallergy.2016.6.2.75.Human leukocyte antigen-associated severe cutaneous adverse drug reactions: from bedside to bench and beyond
Dinh Van Nguyen, Christopher Vidal, Hieu Chi Chu, Sheryl van Nunen
Asia Pac Allergy. 2019;9(3):. doi: 10.5415/apallergy.2019.9.e20.
Reference
-
1. Wheatley LM, Plaut M, Schwaninger JM, Banerji A, Castells M, Finkelman FD, Gleich GJ, Guttman-Yassky E, Mallal SA, Naisbitt DJ, Ostrov DA, Phillips EJ, Pichler WJ, Platts-Mills TA, Roujeau JC, Schwartz LB, Trepanier LA. Report from the National Institute of Allergy and Infectious Diseases workshop on drug allergy. J Allergy Clin Immunol. 2015; 136:262–271.e2.
Article2. Greenberger PA. 8. Drug allergy. J Allergy Clin Immunol. 2006; 117:2 Suppl Mini-Primer. S464–S470.
Article3. Thong BY, Tan TC. Epidemiology and risk factors for drug allergy. Br J Clin Pharmacol. 2011; 71:684–700.
Article4. Landsteiner K, Jacobs J. Studies on the sensitization of animals with simple chemical compounds. II. J Exp Med. 1936; 64:625–639.
Article5. Landsteiner K, Jacobs J. Studies on the sensitization of animals with simple chemical compounds : III. Anaphylaxis induced by arsphenamine. J Exp Med. 1936; 64:717–721.6. LevinE BB. Studies on the mechanism of the formation of the penicillin antigen. I. Delayed allergic cross-reactions among penicillin G and its degradation products. J Exp Med. 1960; 112:1131–1156.7. Ariza A, Mayorga C, Fernandez TD, Barbero N, Martín-Serrano A, Pérez-Sala D, Sánchez-Gómez FJ, Blanca M, Torres MJ, Montanez MI. Hypersensitivity reactions to β-lactams: relevance of hapten-protein conjugates. J Investig Allergol Clin Immunol. 2015; 25:12–25.8. Whitaker P, Meng X, Lavergne SN, El-Ghaiesh S, Monshi M, Earnshaw C, Peckham D, Gooi J, Conway S, Pirmohamed M, Jenkins RE, Naisbitt DJ, Park BK. Mass spectrometric characterization of circulating and functional antigens derived from piperacillin in patients with cystic fibrosis. J Immunol. 2011; 187:200–211.
Article9. Monshi MM, Faulkner L, Gibson A, Jenkins RE, Farrell J, Earnshaw CJ, Alfirevic A, Cederbrant K, Daly AK, French N, Pirmohamed M, Park BK, Naisbitt DJ. Human leukocyte antigen (HLA)-B*57:01-restricted activation of drug-specific T cells provides the immunological basis for flucloxacillin-induced liver injury. Hepatology. 2013; 57:727–739.10. Meng X, Jenkins RE, Berry NG, Maggs JL, Farrell J, Lane CS, Stachulski AV, French NS, Naisbitt DJ, Pirmohamed M, Park BK. Direct evidence for the formation of diastereoisomeric benzylpenicilloyl haptens from benzylpenicillin and benzylpenicillenic acid in patients. J Pharmacol Exp Ther. 2011; 338:841–849.
Article11. Levine BB, Ovary Z. Studies on the mechanism of the formation of the penicillin antigen. III. The N-(D-alpha-benzylpenicilloyl) group as an antigenic determinant responsible for hypersensitivity to penicillin G. J Exp Med. 1961; 114:875–904.12. Batchelor FR, Dewdney JM, Gazzard D. Penicillin allergy: the formation of the penicilloyl determinant. Nature. 1965; 206:362–364.
Article13. Jenkins RE, Meng X, Elliott VL, Kitteringham NR, Pirmohamed M, Park BK. Characterisation of flucloxacillin and 5-hydroxymethyl flucloxacillin haptenated HSA in vitro and in vivo. Proteomics Clin Appl. 2009; 3:720–729.
Article14. Callan HE, Jenkins RE, Maggs JL, Lavergne SN, Clarke SE, Naisbitt DJ, Park BK. Multiple adduction reactions of nitroso sulfamethoxazole with cysteinyl residues of peptides and proteins: implications for hapten formation. Chem Res Toxicol. 2009; 22:937–948.
Article15. Sanderson JP, Naisbitt DJ, Farrell J, Ashby CA, Tucker MJ, Rieder MJ, Pirmohamed M, Clarke SE, Park BK. Sulfamethoxazole and its metabolite nitroso sulfamethoxazole stimulate dendritic cell costimulatory signaling. J Immunol. 2007; 178:5533–5542.
Article16. Naisbitt DJ, Farrell J, Gordon SF, Maggs JL, Burkhart C, Pichler WJ, Pirmohamed M, Park BK. Covalent binding of the nitroso metabolite of sulfamethoxazole leads to toxicity and major histocompatibility complex-restricted antigen presentation. Mol Pharmacol. 2002; 62:628–637.
Article17. Pickard C, Smith AM, Cooper H, Strickland I, Jackson J, Healy E, Friedmann PS. Investigation of mechanisms underlying the T-cell response to the hapten 2,4-dinitrochlorobenzene. J Invest Dermatol. 2007; 127:630–637.
Article18. El-Ghaiesh S, Monshi MM, Whitaker P, Jenkins R, Meng X, Farrell J, Elsheikh A, Peckham D, French N, Pirmohamed M, Park BK, Naisbitt DJ. Characterization of the antigen specificity of T-cell clones from piperacillin-hypersensitive patients with cystic fibrosis. J Pharmacol Exp Ther. 2012; 341:597–610.
Article19. Zanni MP, von Greyerz S, Schnyder B, Brander KA, Frutig K, Hari Y, Valitutti S, Pichler WJ. HLA-restricted, processing- and metabolism-independent pathway of drug recognition by human alpha beta T lymphocytes. J Clin Invest. 1998; 102:1591–1598.
Article20. Pichler WJ. Delayed drug hypersensitivity reactions. Ann Intern Med. 2003; 139:683–693.
Article21. Schnyder B, Mauri-Hellweg D, Zanni M, Bettens F, Pichler WJ. Direct, MHC-dependent presentation of the drug sulfamethoxazole to human alphabeta T cell clones. J Clin Invest. 1997; 100:136–141.
Article22. Keller M, Lerch M, Britschgi M, Tache V, Gerber BO, Lüthi M, Lochmatter P, Kanny G, Bircher AJ, Christiansen C, Pichler WJ. Processing-dependent and -independent pathways for recognition of iodinated contrast media by specific human T cells. Clin Exp Allergy. 2010; 40:257–268.
Article23. Naisbitt DJ, Farrell J, Wong G, Depta JP, Dodd CC, Hopkins JE, Gibney CA, Chadwick DW, Pichler WJ, Pirmohamed M, Park BK. Characterization of drug-specific T cells in lamotrigine hypersensitivity. J Allergy Clin Immunol. 2003; 111:1393–1403.
Article24. Adam J, Pichler WJ, Yerly D. Delayed drug hypersensitivity: models of T-cell stimulation. Br J Clin Pharmacol. 2011; 71:701–707.
Article25. Pichler WJ, Adam J, Watkins S, Wuillemin N, Yun J, Yerly D. Drug hypersensitivity: how drugs stimulate T cells via pharmacological interaction with immune receptors. Int Arch Allergy Immunol. 2015; 168:13–24.
Article26. Watkins S, Pichler WJ. Sulfamethoxazole induces a switch mechanism in T cell receptors containing TCRVβ20-1, altering pHLA recognition. PLoS One. 2013; 8:e76211.
Article27. Yun J, Marcaida MJ, Eriksson KK, Jamin H, Fontana S, Pichler WJ, Yerly D. Oxypurinol directly and immediately activates the drug-specific T cells via the preferential use of HLA-B*58:01. J Immunol. 2014; 192:2984–2993.28. Illing PT, Vivian JP, Dudek NL, Kostenko L, Chen Z, Bharadwaj M, Miles JJ, Kjer-Nielsen L, Gras S, Williamson NA, Burrows SR, Purcell AW, Rossjohn J, McCluskey J. Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature. 2012; 486:554–558.
Article29. Ostrov DA, Grant BJ, Pompeu YA, Sidney J, Harndahl M, Southwood S, Oseroff C, Lu S, Jakoncic J, de Oliveira CA, Yang L, Mei H, Shi L, Shabanowitz J, English AM, Wriston A, Lucas A, Phillips E, Mallal S, Grey HM, Sette A, Hunt DF, Buus S, Peters B. Drug hypersensitivity caused by alteration of the MHC-presented self-peptide repertoire. Proc Natl Acad Sci U S A. 2012; 109:9959–9964.
Article30. Adam J, Wuillemin N, Watkins S, Jamin H, Eriksson KK, Villiger P, Fontana S, Pichler WJ, Yerly D. Abacavir induced T cell reactivity from drug naïve individuals shares features of allo-immune responses. PLoS One. 2014; 9:e95339.
Article31. Watkins S, Pichler WJ. Activating interactions of sulfanilamides with T cell receptors. Open J Immunol. 2013; 3:139–157.
Article32. Wuillemin N, Adam J, Fontana S, Krähenbühl S, Pichler WJ, Yerly D. HLA haplotype determines hapten or p-i T cell reactivity to flucloxacillin. J Immunol. 2013; 190:4956–4964.
Article33. Adam J, Eriksson KK, Schnyder B, Fontana S, Pichler WJ, Yerly D. Avidity determines T-cell reactivity in abacavir hypersensitivity. Eur J Immunol. 2012; 42:1706–1716.
Article34. Burkhart C, Britschgi M, Strasser I, Depta JP, von Greyerz S, Barnaba V, Pichler WJ. Non-covalent presentation of sulfamethoxazole to human CD4+ T cells is independent of distinct human leucocyte antigen-bound peptides. Clin Exp Allergy. 2002; 32:1635–1643.
Article35. Zanni MP, von Greyerz S, Schnyder B, Wendland T, Pichler WJ. Allele-unrestricted presentation of lidocaine by HLA-DR molecules to specific alphabeta+ T cell clones. Int Immunol. 1998; 10:507–515.
Article36. Ko TM, Chung WH, Wei CY, Shih HY, Chen JK, Lin CH, Chen YT, Hung SI. Shared and restricted T-cell receptor use is crucial for carbamazepine-induced Stevens-Johnson syndrome. J Allergy Clin Immunol. 2011; 128:1266–1276.e11.
Article37. Wei CY, Chung WH, Huang HW, Chen YT, Hung SI. Direct interaction between HLA-B and carbamazepine activates T cells in patients with Stevens-Johnson syndrome. J Allergy Clin Immunol. 2012; 129:1562–1569.e5.
Article38. Zhou P, Zhang S, Wang Y, Yang C, Huang J. Structural modeling of HLA-B*1502/peptide/carbamazepine/T-cell receptor complex architecture: implication for the molecular mechanism of carbamazepine-induced Stevens-Johnson syndrome/toxic epidermal necrolysis. J Biomol Struct Dyn. 2015; 12. 14. [Epub]. DOI: 10.1080/07391102.2015.1092476.39. Illing PT, Vivian JP, Purcell AW, Rossjohn J, McCluskey J. Human leukocyte antigen-associated drug hypersensitivity. Curr Opin Immunol. 2013; 25:81–89.
Article40. Yang L, Chen J, He L. Harvesting candidate genes responsible for serious adverse drug reactions from a chemical-protein interactome. PLoS Comput Biol. 2009; 5:e1000441.
Article41. Kirchhof MG, Miliszewski MA, Sikora S, Papp A, Dutz JP. Retrospective review of Stevens-Johnson syndrome/toxic epidermal necrolysis treatment comparing intravenous immunoglobulin with cyclosporine. J Am Acad Dermatol. 2014; 71:941–947.
Article42. Valeyrie-Allanore L, Wolkenstein P, Brochard L, Ortonne N, Maître B, Revuz J, Bagot M, Roujeau JC. Open trial of ciclosporin treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2010; 163:847–853.
Article43. Chung WH, Chang WC, Stocker SL, Juo CG, Graham GG, Lee MH, Williams KM, Tian YC, Juan KC, Jan Wu YJ, Yang CH, Chang CJ, Lin YJ, Day RO, Hung SI. Insights into the poor prognosis of allopurinol-induced severe cutaneous adverse reactions: the impact of renal insufficiency, high plasma levels of oxypurinol and granulysin. Ann Rheum Dis. 2015; 74:2157–2164.
Article44. Garcia-Doval I, LeCleach L, Bocquet H, Otero XL, Roujeau JC. Toxic epidermal necrolysis and Stevens-Johnson syndrome: does early withdrawal of causative drugs decrease the risk of death? Arch Dermatol. 2000; 136:323–327.
Article45. Yun J, Mattsson J, Schnyder K, Fontana S, Largiadèr CR, Pichler WJ, Yerly D. Allopurinol hypersensitivity is primarily mediated by dose-dependent oxypurinol-specific T cell response. Clin Exp Allergy. 2013; 43:1246–1255.
Article46. Norcross MA, Luo S, Lu L, Boyne MT, Gomarteli M, Rennels AD, Woodcock J, Margulies DH, McMurtrey C, Vernon S, Hildebrand WH, Buchli R. Abacavir induces loading of novel self-peptides into HLA-B*57: 01: an autoimmune model for HLA-associated drug hypersensitivity. AIDS. 2012; 26:F21–F29.47. Hughes AR, Mosteller M, Bansal AT, Davies K, Haneline SA, Lai EH, Nangle K, Scott T, Spreen WR, Warren LL, Roses AD. CNA30027 Study Team. CNA30032 Study Team. Association of genetic variations in HLA-B region with hypersensitivity to abacavir in some, but not all, populations. Pharmacogenomics. 2004; 5:203–211.
Article48. Mallal S, Phillips E, Carosi G, Molina JM, Workman C, Tomazic J, Jägel-Guedes E, Rugina S, Kozyrev O, Cid JF, Hay P, Nolan D, Hughes S, Hughes A, Ryan S, Fitch N, Thorborn D, Benbow A. PREDICT-1 Study Team. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008; 358:568–579.49. Lucas A, Lucas M, Strhyn A, Keane NM, McKinnon E, Pavlos R, Moran EM, Meyer-Pannwitt V, Gaudieri S, D'Orsogna L, Kalams S, Ostrov DA, Buus S, Peters B, Mallal S, Phillips E. Abacavir-reactive memory T cells are present in drug naïve individuals. PLoS One. 2015; 10:e0117160.
Article50. Metushi IG, Wriston A, Banerjee P, Gohlke BO, English AM, Lucas A, Moore C, Sidney J, Buus S, Ostrov DA, Mallal S, Phillips E, Shabanowitz J, Hunt DF, Preissner R, Peters B. Acyclovir has low but detectable influence on HLA-B*57:01 specificity without inducing hypersensitivity. PLoS One. 2015; 10:e0124878.51. Chessman D, Kostenko L, Lethborg T, Purcell AW, Williamson NA, Chen Z, Kjer-Nielsen L, Mifsud NA, Tait BD, Holdsworth R, Almeida CA, Nolan D, Macdonald WA, Archbold JK, Kellerher AD, Marriott D, Mallal S, Bharadwaj M, Rossjohn J, McCluskey J. Human leukocyte antigen class I-restricted activation of CD8+ T cells provides the immunogenetic basis of a systemic drug hypersensitivity. Immunity. 2008; 28:822–832.
Article52. Castrejon JL, Berry N, El-Ghaiesh S, Gerber B, Pichler WJ, Park BK, Naisbitt DJ. Stimulation of human T cells with sulfonamides and sulfonamide metabolites. J Allergy Clin Immunol. 2010; 125:411–418.e4.
Article53. Brander C, Mauri-Hellweg D, Bettens F, Rolli H, Goldman M, Pichler WJ. Heterogeneous T cell responses to beta-lactam-modified self-structures are observed in penicillin-allergic individuals. J Immunol. 1995; 155:2670–2678.54. Yaseen FS, Saide K, Kim SH, Monshi M, Tailor A, Wood S, Meng X, Jenkins R, Faulkner L, Daly AK, Pirmohamed M, Park BK, Naisbitt DJ. Promiscuous T-cell responses to drugs and drug-haptens. J Allergy Clin Immunol. 2015; 136:474–476.e8.
Article55. Yun J, Adam J, Yerly D, Pichler WJ. Human leukocyte antigens (HLA) associated drug hypersensitivity: consequences of drug binding to HLA. Allergy. 2012; 67:1338–1346.
Article56. Lochmatter P, Beeler A, Kawabata TT, Gerber BO, Pichler WJ. Drug-specific in vitro release of IL-2, IL-5, IL-13 and IFN-gamma in patients with delayed-type drug hypersensitivity. Allergy. 2009; 64:1269–1278.57. Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, Chin SW, Chiou CC, Chu SC, Ho HC, Yang CH, Lu CF, Wu JY, Liao YD, Chen YT. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med. 2008; 14:1343–1350.
Article58. Chung WH, Pan RY, Chu MT, Chin SW, Huang YL, Wang WC, Chang JY, Hung SI. Oxypurinol-specific T cells possess preferential TCR clonotypes and express granulysin in allopurinol-induced severe cutaneous adverse reactions. J Invest Dermatol. 2015; 135:2237–2248.
Article59. Matzinger P. The danger model: a renewed sense of self. Science. 2002; 296:301–305.
Article60. Pradeu T, Cooper EL. The danger theory: 20 years later. Front Immunol. 2012; 3:287.
Article61. Zhang X, Liu F, Chen X, Zhu X, Uetrecht J. Involvement of the immune system in idiosyncratic drug reactions. Drug Metab Pharmacokinet. 2011; 26:47–59.
Article62. Uetrecht J. Idiosyncratic drug reactions: current understanding. Annu Rev Pharmacol Toxicol. 2007; 47:513–539.
Article63. Ellrodt AG, Murata GH, Riedinger MS, Stewart ME, Mochizuki C, Gray R. Severe neutropenia associated with sustained-release procainamide. Ann Intern Med. 1984; 100:197–201.
Article64. Rawlins MD, Thompson JW. Pathogenesis of adverse drug reactions. Oxford: Oxford University Press;1977.65. Phillips EJ, Mallal SA. Pharmacogenetics of drug hypersensitivity. Pharmacogenomics. 2010; 11:973–987.
Article66. Jung JW, Song WJ, Kim YS, Joo KW, Lee KW, Kim SH, Park HW, Chang YS, Cho SH, Min KU, Kang HR. HLA-B58 can help the clinical decision on starting allopurinol in patients with chronic renal insufficiency. Nephrol Dial Transplant. 2011; 26:3567–3572.
Article67. Ko TM, Tsai CY, Chen SY, Chen KS, Yu KH, Chu CS, Huang CM, Wang CR, Weng CT, Yu CL, Hsieh SC, Tsai JC, Lai WT, Tsai WC, Yin GD, Ou TT, Cheng KH, Yen JH, Liou TL, Lin TH, Chen DY, Hsiao PJ, Weng MY, Chen YM, Chen CH, Liu MF, Yen HW, Lee JJ, Kuo MC, Wu CC, Hung SY, Luo SF, Yang YH, Chuang HP, Chou YC, Liao HT, Wang CW, Huang CL, Chang CS, Lee MT, Chen P, Wong CS, Chen CH, Wu JY, Chen YT, Shen CY. Taiwan Allopurinol-SCAR Consortium. Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: national prospective cohort study. BMJ. 2015; 351:h4848.68. Chen P, Lin JJ, Lu CS, Ong CT, Hsieh PF, Yang CC, Tai CT, Wu SL, Lu CH, Hsu YC, Yu HY, Ro LS, Lu CT, Chu CC, Tsai JJ, Su YH, Lan SH, Sung SF, Lin SY, Chuang HP, Huang LC, Chen YJ, Tsai PJ, Liao HT, Lin YH, Chen CH, Chung WH, Hung SI, Wu JY, Chang CF, Chen L, Chen YT, Shen CY. Taiwan SJS Consortium. Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N Engl J Med. 2011; 364:1126–1133.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regulation of IgE-Mediated Food Allergy by IL-9 Producing Mucosal Mast Cells and Type 2 Innate Lymphoid Cells
- Cyclosporine Desensitization in Patient with Multiple Hypersensitivity Reactions Immediately after Peripheral Blood Stem Cell Transplantation
- Diagnosis and Management of Antibiotic Allergies
- Recent progress of elucidating the mechanisms of drug hypersensitivity
- An Overview of Multiple Drug Hypersensitivity Syndrome