Immune Netw.
2016 Aug;16(4):211-218. 10.4110/in.2016.16.4.211.
Regulation of IgE-Mediated Food Allergy by IL-9 Producing Mucosal Mast Cells and Type 2 Innate Lymphoid Cells
- Affiliations
-
- 1Laboratory of Immunology and Infectious Diseases, Graduate School of Medical Science and Engineering, KAIST, Daejeon 34141, Korea. jeeboong.lee@kaist.ac.kr
- KMID: 2349882
- DOI: http://doi.org/10.4110/in.2016.16.4.211
Abstract
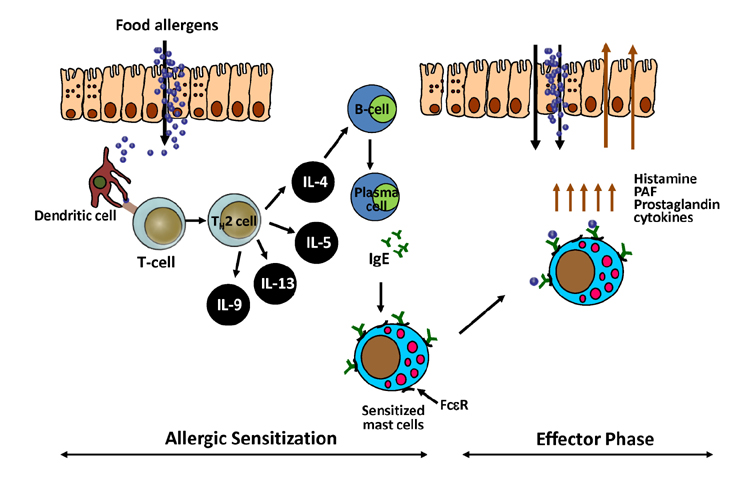
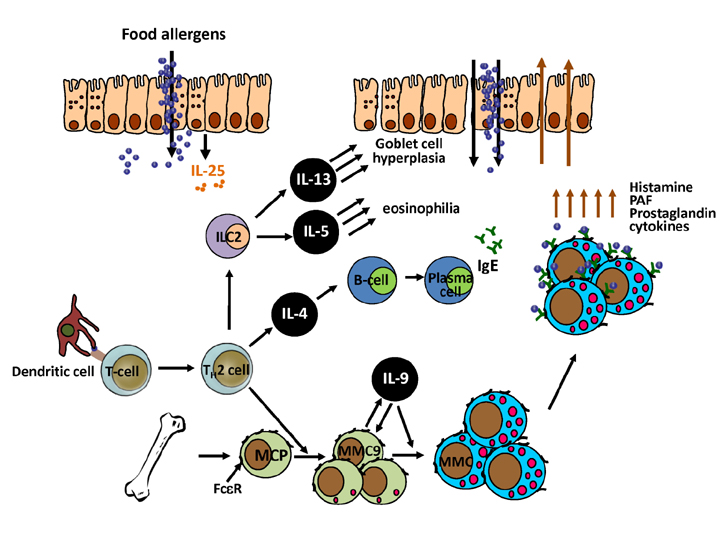
- Due to the increasing prevalence and number of life-threatening cases, food allergy has emerged as a major health concern. The classic immune response seen during food allergy is allergen-specific IgE sensitization and hypersensitivity reactions to foods occur in the effector phase with often severe and deleterious outcomes. Recent research has advanced understanding of the immunological mechanisms occurring during the effector phase of allergic reactions to ingested food. Therefore, this review will not only cover the mucosal immune system of the gastrointestinal tract and the immunological mechanisms underlying IgE-mediated food allergy, but will also introduce cells recently identified to have a role in the hypersensitivity reaction to food allergens. These include IL-9 producing mucosal mast cells (MMC9s) and type 2 innate lymphoid cells (ILC2s). The involvement of these cell types in potentiating the type 2 immune response and developing the anaphylactic response to food allergens will be discussed. In addition, it has become apparent that there is a collaboration between these cells that contributes to an individual's susceptibility to IgE-mediated food allergy.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Regulation of Allergic Immune Responses by Microbial Metabolites
Hyun Jung Park, Sung Won Lee, Seokmann Hong
Immune Netw. 2018;18(1):e15. doi: 10.4110/in.2018.18.e15.Regulation of Allergic Immune Responses by Microbial Metabolites
Hyun Jung Park, Sung Won Lee, Seokmann Hong
Immune Netw. 2018;18(1):. doi: 10.4110/in.2018.18.e15.
Reference
-
1. Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, Motala C, Ortega Martell JA, Platts-Mills TA, Ring J, Thien F, Van CP, Williams HC. Revised nomenclature for allergy for global use: Report of the nomenclature review committee of the world allergy organization, October 2003. J Allergy Clin Immunol. 2004; 113:832–836.
Article2. Platts-Mills TA. The allergy epidemics: 1870-2010. J Allergy Clin Immunol. 2015; 136:3–13.
Article3. Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. 2011; 127:594–602.
Article4. Sicherer SH, Noone SA, Munoz-Furlong A. The impact of childhood food allergy on quality of life. Ann Allergy Asthma Immunol. 2001; 87:461–464.
Article5. Flokstra-de Blok BM, van der Velde JL, Vlieg-Boerstra BJ, Oude Elberink JN, DunnGalvin A, Hourihane JO, Duiverman EJ, Dubois AE. Health-related quality of life of food allergic patients measured with generic and disease-specific questionnaires. Allergy. 2010; 65:1031–1038.
Article6. Lee JB, Chen CY, Liu B, Mugge L, Angkasekwinai P, Facchinetti V, Dong C, Liu YJ, Rothenberg ME, Hogan SP, Finkelman FD, Wang YH. IL-25 and CD4(+) TH2 cells enhance type 2 innate lymphoid cell-derived IL-13 production, which promotes IgE-mediated experimental food allergy. J Allergy Clin Immunol. 2016; 137:1216–1225.
Article7. Chen CY, Lee JB, Liu B, Ohta S, Wang PY, Kartashov AV, Mugge L, Abonia JP, Barski A, Izuhara K, Rothenberg ME, Finkelman FD, Hogan SP, Wang YH. Induction of interleukin-9-producing mucosal mast cells promotes susceptibility to IgE-mediated experimental food allergy. Immunity. 2015; 43:788–802.
Article8. Brandtzaeg P. Development and basic mechanisms of human gut immunity. Nutr Rev. 1998; 56:S5–S18.
Article9. Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012; 489:220–230.
Article10. Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009; 9:799–809.
Article11. Nagler-Anderson C. Man the barrier! Strategic defences in the intestinal mucosa. Nat Rev Immunol. 2001; 1:59–67.
Article12. Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjoberg J, Amir E, Teggatz P, Barman M, Hayward M, Eastwood D, Stoel M, Zhou Y, Sodergren E, Weinstock GM, Bevins CL, Williams CB, Bos NA. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010; 11:76–83.
Article13. Moog F. The lining of the small intestine. Sci Am. 1981; 245:154–158. 160–162.
Article14. Chase MW. Inhibition of experimental drug allergy by prior feeding of the sensitizing agent. Proc Soc Exp Biol Med. 1946; 61:257–259.
Article15. Iweala OI, Nagler CR. Immune privilege in the gut: the establishment and maintenance of non-responsiveness to dietary antigens and commensal flora. Immunol Rev. 2006; 213:82–100.
Article16. Muraro A, Dubois AE, DunnGalvin A, Hourihane JO, de Jong NW, Meyer R, Panesar SS, Roberts G, Salvilla S, Sheikh A, Worth A, Flokstra-de Blok BM. EAACI Food allergy and anaphylaxis guidelines Food allergy health-related quality of life measures. Allergy. 2014; 69:845–853.
Article17. Liu T, Navarro S, Lopata AL. Current advances of murine models for food allergy. Mol Immunol. 2016; 70:104–117.
Article18. Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, Zimmermann N, Finkelman FD, Rothenberg ME. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest. 2003; 112:1666–1677.
Article19. Elson CO, Ealding W. Cholera toxin feeding did not induce oral tolerance in mice and abrogated oral tolerance to an unrelated protein antigen. J Immunol. 1984; 133:2892–2897.20. Kweon MN, Yamamoto M, Kajiki M, Takahashi I, Kiyono H. Systemically derived large intestinal CD4(+) Th2 cells play a central role in STAT6-mediated allergic diarrhea. J Clin Invest. 2000; 106:199–206.
Article21. Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, Chu C, Quelle FW, Nosaka T, Vignali DA, Doherty PC, Grosveld G, Paul WE, Ihle JN. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996; 380:630–633.
Article22. Forbes EE, Groschwitz K, Abonia JP, Brandt EB, Cohen E, Blanchard C, Ahrens R, Seidu L, McKenzie A, Strait R, Finkelman FD, Foster PS, Matthaei KI, Rothenberg ME, Hogan SP. IL-9- and mast cellmediated intestinal permeability predisposes to oral antigen hypersensitivity. J Exp Med. 2008; 205:897–913.
Article23. Metcalfe DD, Peavy RD, Gilfillan AM. Mechanisms of mast cell signaling in anaphylaxis. J Allergy Clin Immunol. 2009; 124:639–646.
Article24. Knol EF. Requirements for effective IgE cross-linking on mast cells and basophils. Mol Nutr Food Res. 2006; 50:620–624.
Article25. Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012; 18:693–704.
Article26. Williams LW, Bock SA. Skin testing and food challenges in allergy and immunology practice. Clin Rev Allergy Immunol. 1999; 17:323–338.
Article27. Wang J, Sampson HA. Food anaphylaxis. Clin Exp Allergy. 2007; 37:651–660.
Article28. Rescigno M, Rotta G, Valzasina B, Ricciardi-Castagnoli P. Dendritic cells shuttle microbes across gut epithelial monolayers. Immunobiology. 2001; 204:572–581.
Article29. Blanas E, Davey GM, Carbone FR, Heath WR. A bone marrow-derived APC in the gut-associated lymphoid tissue captures oral antigens and presents them to both CD4+ and CD8+ T cells. J Immunol. 2000; 164:2890–2896.
Article30. Huang FP, Platt N, Wykes M, Major JR, Powell TJ, Jenkins CD, MacPherson GG. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med. 2000; 191:435–444.
Article31. Martin E, O'Sullivan B, Low P, Thomas R. Antigen-specific suppression of a primed immune response by dendritic cells mediated by regulatory T cells secreting interleukin-10. Immunity. 2003; 18:155–167.
Article32. Kweon MN, Fujihashi K, Wakatsuki Y, Koga T, Yamamoto M, McGhee JR, Kiyono H. Mucosally induced systemic T cell unresponsiveness to ovalbumin requires CD40 ligand-CD40 interactions. J Immunol. 1999; 162:1904–1909.33. Noval Rivas M, Burton OT, Wise P, Charbonnier LM, Georgiev P, Oettgen HC, Rachid R, Chatila TA. Regulatory T cell reprogramming toward a Th2-cell-like lineage impairs oral tolerance and promotes food allergy. Immunity. 2015; 42:512–523.
Article34. Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005; 6:345–352.
Article35. Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, McIlgorm A, Jolin HE, McKenzie AN. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006; 203:1105–1116.
Article36. Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, Hepworth MR, Van Voorhees AS, Comeau MR, Artis D. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013; 5:170ra16.
Article37. Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, Huang LC, Johnson D, Scanlon ST, McKenzie AN, Fallon PG, Ogg GS. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013; 210:2939–2950.
Article38. Wang YH, Liu YJ. Thymic stromal lymphopoietin, OX40-ligand, and interleukin-25 in allergic responses. Clin Exp Allergy. 2009; 39:798–806.
Article39. Wang Q, Du J, Zhu J, Yang X, Zhou B. Thymic stromal lymphopoietin signaling in CD4(+) T cells is required for TH2 memory. J Allergy Clin Immunol. 2015; 135:781–791.e3.
Article40. Iijima K, Kobayashi T, Hara K, Kephart GM, Ziegler SF, McKenzie AN, Kita H. IL-33 and thymic stromal lymphopoietin mediate immune pathology in response to chronic airborne allergen exposure. J Immunol. 2014; 193:1549–1559.
Article41. Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF Jr, Tocker JE, Budelsky AL, Kleinschek MA, Kastelein RA, Kambayashi T, Bhandoola A. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010; 464:1362–1366.
Article42. Owyang AM, Zaph C, Wilson EH, Guild KJ, McClanahan T, Miller HR, Cua DJ, Goldschmidt M, Hunter CA, Kastelein RA, Artis D. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J Exp Med. 2006; 203:843–849.
Article43. Angkasekwinai P, Park H, Wang YH, Wang YH, Chang SH, Corry DB, Liu YJ, Zhu Z, Dong C. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. 2007; 204:1509–1517.
Article44. Muto T, Fukuoka A, Kabashima K, Ziegler SF, Nakanishi K, Matsushita K, Yoshimoto T. The role of basophils and proallergic cytokines, TSLP and IL-33, in cutaneously sensitized food allergy. Int Immunol. 2014; 26:539–549.
Article45. McKenzie GJ, Emson CL, Bell SE, Anderson S, Fallon P, Zurawski G, Murray R, Grencis R, McKenzie AN. Impaired development of Th2 cells in IL-13-deficient mice. Immunity. 1998; 9:423–432.
Article46. Wu D, Ahrens R, Osterfeld H, Noah TK, Groschwitz K, Foster PS, Steinbrecher KA, Rothenberg ME, Shroyer NF, Matthaei KI, Finkelman FD, Hogan SP. Interleukin-13 (IL-13)/IL-13 receptor alpha1 (IL-13Ralpha1) signaling regulates intestinal epithelial cystic fibrosis transmembrane conductance regulator channel-dependent Cl-secretion. J Biol Chem. 2011; 286:13357–13369.
Article47. Gurish MF, Austen KF. Developmental origin and functional specialization of mast cell subsets. Immunity. 2012; 37:25–33.
Article48. Osterfeld H, Ahrens R, Strait R, Finkelman FD, Renauld JC, Hogan SP. Differential roles for the IL-9/IL-9 receptor alpha-chain pathway in systemic and oral antigen-induced anaphylaxis. J Allergy Clin Immunol. 2010; 125:469–476.e2.49. Ahrens R, Osterfeld H, Wu D, Chen CY, Arumugam M, Groschwitz K, Strait R, Wang YH, Finkelman FD, Hogan SP. Intestinal mast cell levels control severity of oral antigen-induced anaphylaxis in mice. Am J Pathol. 2012; 180:1535–1546.
Article50. Steenwinckel V, Louahed J, Lemaire MM, Sommereyns C, Warnier G, McKenzie A, Brombacher F, Van SJ, Renauld JC. IL-9 promotes IL-13-dependent paneth cell hyperplasia and up-regulation of innate immunity mediators in intestinal mucosa. J Immunol. 2009; 182:4737–4743.
Article51. Brough HA, Cousins DJ, Munteanu A, Wong YF, Sudra A, Makinson K, Stephens AC, Arno M, Ciortuz L, Lack G, Turcanu V. IL-9 is a key component of memory TH cell peanut-specific responses from children with peanut allergy. J Allergy Clin Immunol. 2014; 134:1329–1338.e10.
Article52. Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor-beta 'reprograms' the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008; 9:1341–1346.
Article53. Stassen M, Arnold M, Hultner L, Muller C, Neudorfl C, Reineke T, Schmitt E. Murine bone marrow-derived mast cells as potent producers of IL-9: costimulatory function of IL-10 and kit ligand in the presence of IL-1. J Immunol. 2000; 164:5549–5555.
Article54. Shimbara A, Christodoulopoulos P, Soussi-Gounni A, Olivenstein R, Nakamura Y, Levitt RC, Nicolaides NC, Holroyd KJ, Tsicopoulos A, Lafitte JJ, Wallaert B, Hamid QA. IL-9 and its receptor in allergic and nonallergic lung disease: increased expression in asthma. J Allergy Clin Immunol. 2000; 105:108–115.
Article55. Abdelilah S, Latifa K, Esra N, Cameron L, Bouchaib L, Nicolaides N, Levitt R, Hamid Q. Functional expression of IL-9 receptor by human neutrophils from asthmatic donors: role in IL-8 release. J Immunol. 2001; 166:2768–2774.
Article56. Barlow JL, McKenzie AN. Type-2 innate lymphoid cells in human allergic disease. Curr Opin Allergy Clin Immunol. 2014; 14:397–403.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Type 2 Innate Lymphoid Cells in Allergic Diseases
- Role of Mast Cells in Allergic Inflammation and Innate Immunity
- Heterogeneity of IL-22-producing Lymphoid Tissue Inducer-like Cells in Human and Mouse
- Interleukin-33 and Mast Cells Bridge Innate and Adaptive Immunity: From the Allergologist's Perspective
- The Role of Cytokines in Allergy