Asia Pac Allergy.
2016 Jan;6(1):48-55. 10.5415/apallergy.2016.6.1.48.
Acute and chronic exposure to Tyrophagus putrescentiae induces allergic pulmonary response in a murine model
- Affiliations
-
- 1Laboratory of Pediatric Respirology, Infant Center, Institute of Biomedical Research, PontifÃcia Universidade Católica do Rio Grande do Sul (PUCRS), Porto Alegre 90610-000, Brazil. aline.cunha@pucrs.br
- 2Laboratory of Parasitology, Universidade Regional Integrada do Alto Uruguai e das Missões, Santiago 97700-000, Brazil.
- 3Laboratory of Molecular Parasitology, Institute of Biomedical Research, PUCRS, Porto Alegre 90610-000, Brazil.
- 4Laboratory of Acarology, Natural Science Museum, Centro Universitário UNIVATES, Lajeado 95900-000, Brazil.
- KMID: 2396970
- DOI: http://doi.org/10.5415/apallergy.2016.6.1.48
Abstract
- BACKGROUND
Tyrophagus putrescentiae (Tp) is a source of aeroallergen that causes allergic diseases.
OBJECTIVE
To describe an acute and chronic murine model of allergic asthma with Tp extract with no systemic sensitization and no use of adjuvant.
METHODS
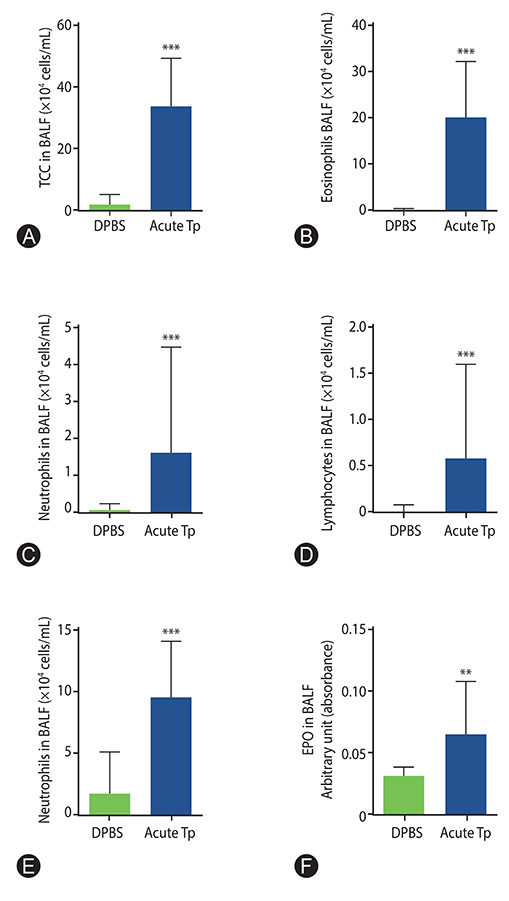
Mites from dust sample were cultured and a raw extract was produced. Female BALB/c mice (6-8 weeks) were challenged intranasally with Tp extract or Dulbecco's phosphate-buffered saline, for 10 consecutive days (acute protocol) or for 6 weeks (chronic protocol). Twenty-four hours after the last intranasal challenge, bronchoalveolar lavage fluid (BALF) was performed for total and differential cells count, cytokine analysis, and eosinophil peroxidase activity. Lung tissue was also removed for histopathologic analysis.
RESULTS
Tp extract has shown a significant increase in total cells count from BALF as well as an increase in absolute eosinophils count, eosinophil peroxidase activity, interleukin (IL)-5 and IL-13 levels, in both acute and chronic protocols. Peribronchovascular infiltrate, goblet cells hyperplasia and collagen deposition were shown in the airways of acute and chronic Tp-exposed mice.
CONCLUSION
Our data suggest that the intranasal exposure to Tp extract, with no systemic sensitization and no use of adjuvants, induces a robust allergic inflammation in the lungs of mice, in both acute and chronic models. Our Tp extract seems to be a potent allergen extract which may be used in asthma model studies.
Keyword
MeSH Terms
Figure
Reference
-
1. Braman SS. The global burden of asthma. Chest. 2006; 130:1 Suppl. 4S–12S.
Article2. Herbert C, Siegle JS, Shadie AM, Nikolaysen S, Garthwaite L, Hansbro NG, Foster PS, Kumar RK. Development of asthmatic inflammation in mice following early-life exposure to ambient environmental particulates and chronic allergen challenge. Dis Model Mech. 2013; 6:479–488.
Article3. Ishmael FT. The inflammatory response in the pathogenesis of asthma. J Am Osteopath Assoc. 2011; 111:11 Suppl 7. S11–S17.4. Panganiban RP, Pinkerton MH, Maru SY, Jefferson SJ, Roff AN, Ishmael FT. Differential microRNA epression in asthma and the role of miR-1248 in regulation of IL-5. Am J Clin Exp Immunol. 2012; 1:154–165.5. Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008; 8:183–192.
Article6. Araujo MI, Junior MM, Cardoso LS, Oliveira RR, Carvalho EM. Sistema de regulacao da resposta imune alérgica. Gaz Méd Bahia. 2008; 78(Suplemento 2):18–25.7. Koyasu S, Moro K. Type 2 innate immune responses and the natural helper cell. Immunology. 2011; 132:475–481.
Article8. Cates EC, Fattouh R, Wattie J, Inman MD, Goncharova S, Coyle AJ, Gutierrez-Ramos JC, Jordana M. Intranasal exposure of mice to house dust mite elicits allergic airway inflammation via a GM-CSF-mediated mechanism. J Immunol. 2004; 173:6384–6392.
Article9. Rydell-Törmanen K, Johnson JR, Fattouh R, Jordana M, Erjefalt JS. Induction of vascular remodeling in the lung by chronic house dust mite exposure. Am J Respir Cell Mol Biol. 2008; 39:61–67.
Article10. Gualdi LP, Pereira AC, Masiero L, Nunez NK, Cao R, Pitrez PM. Murine models for asthma research: an updated critical analysis. Sci Med (Porto Alegre). 2010; 20:236–242.11. Dust mite allergens and asthma: a worldwide problem. J Allergy Clin Immunol. 1989; 83(2 Pt 1):416–427.12. Caraballo L, Puerta L, Martinez B, Moreno L. Identification of allergens from the mite Blomia tropicalis. Clin Exp Allergy. 1994; 24:1056–1060.13. Liao EC, Ho CM, Yin SC, Tsai JJ. Immune responses to tyrophagus putrescentiae-induced airway inflammation in mice. J Investig Allergol Clin Immunol. 2013; 23:20–29.14. Fernandez-Caldas E, Puerta L, Caraballo L. Mites and allergy. Chem Immunol Allergy. 2014; 100:234–242.15. Yu SJ, Liao EC, Tsai JJ. House dust mite allergy: environment evaluation and disease prevention. Asia Pac Allergy. 2014; 4:241–252.
Article16. Iglesias-Souto J, Sanchez-Machin I, Iraola V, Poza P, Gonzalez R, Matheu V. Oral mite anaphylaxis by Thyreophagus entomophagus in a child: a case report. Clin Mol Allergy. 2009; 7:10.
Article17. Liao EC, Lin YH, Chiu CL, Lin TC, Tsai JJ. Identification of allergenic component Tyr p 8 from Tyrophagus putrescentiae and cross-reactivity with Der p 8. Clin Vaccine Immunol. 2013; 20:506–512.
Article18. Arlian LG, Geis DP, Vyszenski-Moher DL, Bernstein IL, Gallagher JS. Antigenic and allergenic properties of the storage mite Tyrophagus putrescentiae. J Allergy Clin Immunol. 1984; 74:166–171.
Article19. Silveira MR, Nunes KP, Cara DC, Souza DG, Correa A Jr, Teixeira MM, Negrao-Correa D. Infection with Strongyloides venezuelensis induces transient airway eosinophilic inflammation, an increase in immunoglobulin E, and hyperresponsiveness in rats. Infect Immun. 2002; 70:6263–6272.20. Park JW, Ko SH, Yong TS, Ree HI, Jeoung BJ, Hong CS. Cross-reactivity of Tyrophagus putrescentiae with Dermatophagoides farinae and Dermatophagoides pteronyssinus in urban areas. Ann Allergy Asthma Immunol. 1999; 83(6 Pt 1):533–539.
Article21. Johnson JR, Wiley RE, Fattouh R, Swirski FK, Gajewska BU, Coyle AJ, Gutierrez-Ramos JC, Ellis R, Inman MD, Jordana M. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am J Respir Crit Care Med. 2004; 169:378–385.
Article22. Ulrich K, Hincks JS, Walsh R, Wetterstrand EM, Fidock MD, Sreckovic S, Lamb DJ, Douglas GJ, Yeadon M, Perros-Huguet C, Evans SM. Anti-inflammatory modulation of chronic airway inflammation in the murine house dust mite model. Pulm Pharmacol Ther. 2008; 21:637–647.
Article23. Hongjia L, Qingling G, Meiying L, Weixuan W, Lihong Z, Yongsheng G, Yanli L, Jinxiang W, Liang D. House dust mite regulate the lung inflammation of asthmatic mice through TLR4 pathway in airway epithelial cells. Cell Biochem Funct. 2010; 28:597–603.
Article24. Fattouh R, Midence NG, Arias K, Johnson JR, Walker TD, Goncharova S, Souza KP, Gregory RC Jr, Lonning S, Gauldie J, Jordana M. Transforming growth factor-beta regulates house dust mite-induced allergic airway inflammation but not airway remodeling. Am J Respir Crit Care Med. 2008; 177:593–603.25. Al-Muhsen S, Johnson JR, Hamid Q. Remodeling in asthma. J Allergy Clin Immunol. 2011; 128:451–462.
Article26. Laitinen LA, Heino M, Laitinen A, Kava T, Haahtela T. Damage of the airway epithelium and bronchial reactivity in patients with asthma. Am Rev Respir Dis. 1985; 131:599–606.
Article27. Saglani S, Mathie SA, Gregory LG, Bell MJ, Bush A, Lloyd CM. Pathophysiological features of asthma develop in parallel in house dust mite-exposed neonatal mice. Am J Respir Cell Mol Biol. 2009; 41:281–289.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The storage mite Tyrophagus putrescentiae induces greater lung inflammation than house dust mites in mice
- A case of bronchial asthma due to Tyrophagus putrescentiae in a non occupational setting
- Tyrophagus putrescentiae: An imporiant allergen in Daejeon
- Prevalence of Sensitization to Tyrophagus putrescentiae in Children with Allegic Diseases
- Identification of Tyrophagus putrescentiae allergens and evaluation of cross-reactivity with Dermatophagoides pteronyssinus