Nat Prod Sci.
2017 Sep;23(3):222-226. 10.20307/nps.2017.23.3.222.
Comparative Effects of Dietary Quercetin and Rutin in Rats Fed with the Lieber-DeCarli Ethanol Diet
- Affiliations
-
- 1Division of Biomedical Convergence, College of Medical Biosciences, Kangwon National University, Chuncheon 24341, Korea. yschoi@kangwon.ac.kr
- 2Institute of Medical Bioscience, Kangwon National University, Chuncheon 24341, Korea.
- KMID: 2393805
- DOI: http://doi.org/10.20307/nps.2017.23.3.222
Abstract
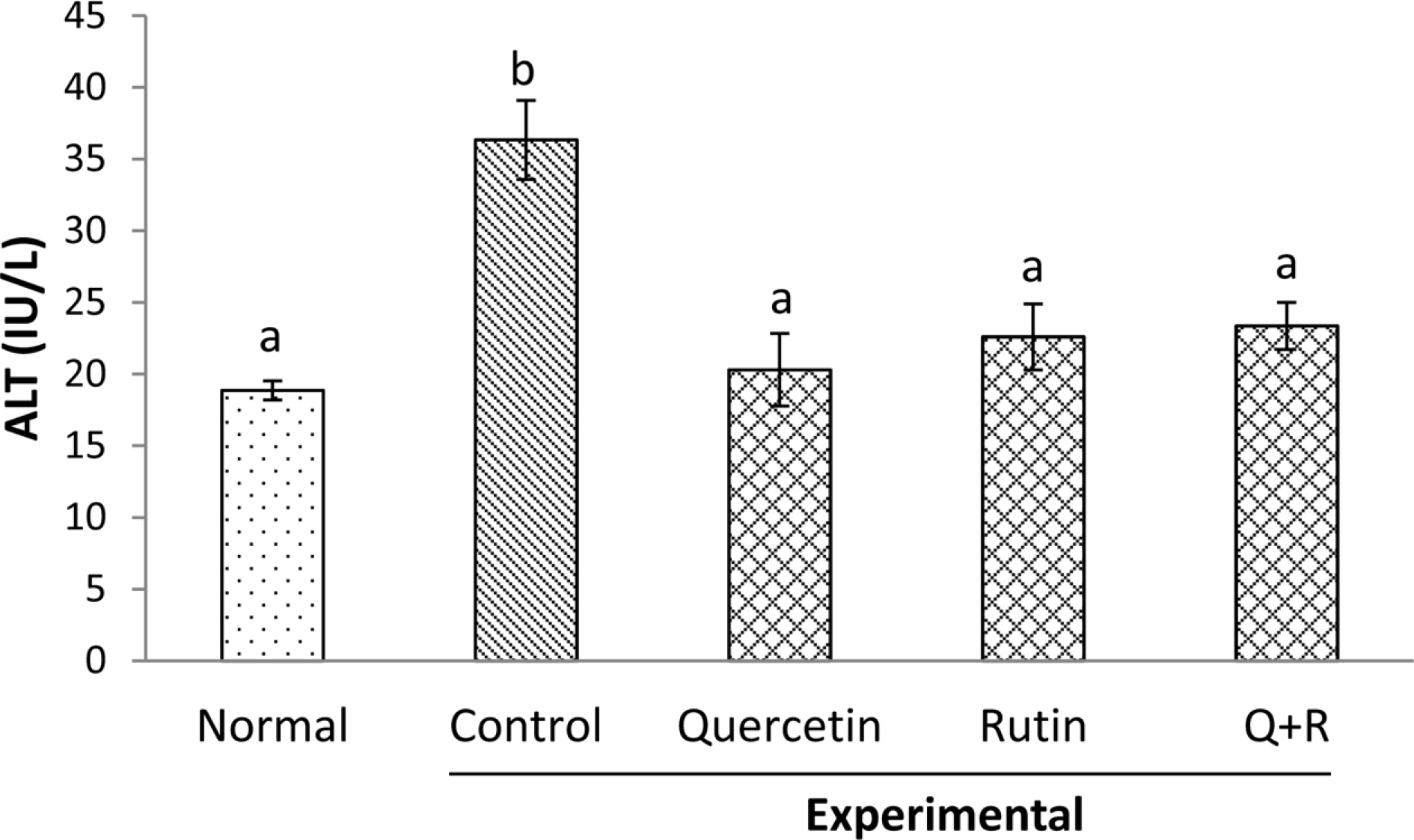
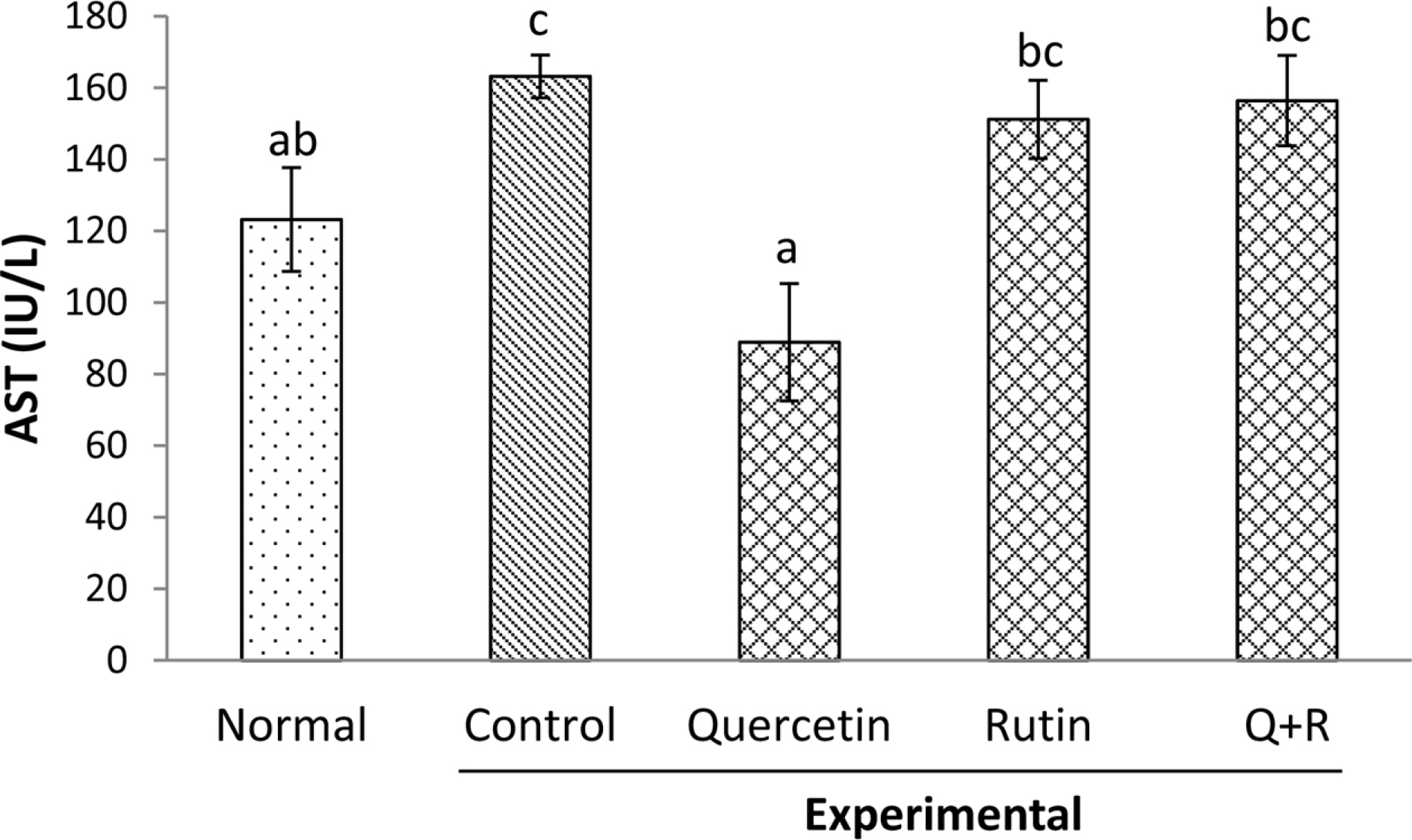
- Flavonoids including quercetin and rutin are a group of naturally occurring compounds widely distributed in plants, especially in buckwheat. Thus, cereal and the leaf of the plant have increasingly used as a source of nutritional and functional foods such as noodle, cake or soup in Korea, Japan and other countries. This study investigated comparative effects of dietary rutin rich in buckwheat and its aglycone, quercetin, on serum biomarkers and antioxidant parameters in rats treated with chronic ethanol. Rats were fed with the liquid diets prepared by the method of Lieber Decarli. Serum alanine transaminase (ALT) and aspartate transaminase (AST) activities increased significantly by alcohol feeding. Dietary flavonoids including rutin, quercetin and their mixtures (1/1, v/v) decreased significantly the activities of serum ALT whereas the feeding of quercetin decreased only the activity of serum AST. The concentration of serum malondialdehydes elevated by chronic alcohol feeding decreased markedly in all the experimental groups that were fed with the flavonoids; however, the combined administration of quercetin or rutin, but not that of rutin or quercetin alone decreased significantly the concentration of liver malondialdehydes to the normal range in rats fed without ethanol. Our results suggested that dietary combined mixture of rutin and quercetin might be effective in ameliorating adverse responses seen in rats exposed to ethanol chronically.
MeSH Terms
Figure
Reference
-
References
(1). Nijveldt R. J.., van Nood E.., van Hoorn D. E.., Boelens P. G.., van Norren K.., van Leeuwen P. A.Am. J. Clin. Nutr. 2001. 74:418–425.(2). Romano B.., Pagano E.., Montanaro V.., Fortunato A. L.., Milic N.., Borrelli F.Phytother. Res. 2013. 27:1588–1596.(3). Boots A. W.., Haenen G. R.., Bast A.Eur. J. Pharmacol. 2008. 585:325–337.(4). Kovalskii I. V.., Krasnyuk I. I.., krasnyuk Jr. I. I.., Nikulina O. I.., Belyatskaya A. V.., Kharitonov Yu. Ya.., Feldman N. B.., Lutsenko S. V.Pharmaceutical Chem. J. 2014. 48:73–76.(5). Crespy V.., Morand C.., Besson C.., Monach C.., Demigne C.., Remesy C. J.Agric. Food Chem. 2002. 50:618–621.(6). Scalbert A.., Morand C.., Monach C.., Rémésy C.Biomed. Pharmacother. 2002. 56:276–282.(7). Carbonaro, M; Grant G.Ann. Nutr. Metab. 2005. 49:178–182.(8). Nordmann R.., Ribière C.., Rouach H.Free Radic. Biol. Med. 1992. 12:219–240.(9). Tang Y.., Gao C.., Xing M.., Li Y.., Zhu L.., Wang D.., Yang X.., Liu L.., Yao P.Food Chem. Toxicol. 2012. 50:1194–1200.(10). Shenbagam M.., Nalini N.Fundam. Clin. Pharmacol. 2011. 25:493–502.(11). Botros M.., Sikaris K.A.Clin. Biochem. Rev. 2013. 34:117–130.(12). Lieber C. S.., DeCarli L. M.Alcohol Alcohol. 1989. 24:197–211.(13). Carr T. P.., Andersen C. J.., Rudel L. L.Clin. Biochem. 1993. 26:39–42.(14). Yagi K.Chem. Phys. Lipids. 1987. 45:337–351.(15). Lowry O. H.., Rosebrough N. J.., Farr A. L.., Randall R. J. J.Biol. Chem. 1951. 193:265–275.(16). Gramenzi A.., Caputo F.., Biselli M.., Kuria F.., Loggi E.., Andreone P.., Bernardi M.Aliment Pharmacol. Ther. 2006. 24:1151–1161.(17). Kim M. K.., Hyun S. H.., Choung S. Y. J.Health Sci. 2006. 52:344–351.(18). Baraona E.., Lieber C. S. J.Lipid Res. 1979. 20:289–315.(19). Zeman F. J.In Clinical Nutrition and Dietetics (2/e): Liver disease and alcoholism; Jones, L Ed; Macmillan Publishing; U.S.A,. 1991. 517–553.(20). Keshavarzian A.., Farhadi A.., Forsyth C. B.., Rangan J.., Jakate S.., Shaikh M.., Banan A.., Fields J. Z. J.Hepatol. 2009. 50:538–547.(21). Nolan J. P.Hepatology. 2010. 52:1829–1835.(22). Wang H. J.., Zakhari S.., Jung M. K.World J. Gastroenterol. 2010. 16:1304–1313.(23). Kim, H; Kong H.., Choi B.., Yang Y.., Kim Y.., Lim M. J.., Neckers L.., Jung Y.Pharm. Res. 2005. 22:1499–1509.(24). Vuppalanchi R.., Juluri R.., Bell L. N.., Ghabril M.., Kamendulis L.., Klaunig J. E.., Saxena R.., Agarwal D.., Johnson M. S.., Chalasani N.Am. J. Med. Sci. 2011. 342:314–317.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of vitamin C and E supplementation on oxidative stress and liver toxicity in rats fed a low-fat ethanol diet
- Effects of Dietary Conjugated Linoleic Acid (CLA)on Antioxidant System in the Liver of Chronically Ethanol -Treated Rats
- Effect of Chronical Ethanol Ingestion on the Levels of Fatty Acid Ethyl Esters (FAEEs) and Lipid Peroxidation in Rat Tissues
- Aspartate modulates the ethanol-induced oxidative stress and glutathione utilizing enzymes in rat testes.
- Iron Supplementation Reverses the Reduction of Hydroxymethylcytosine in Hepatic DNA Associated With Chronic Alcohol Consumption in Rats