J Pathol Transl Med.
2017 May;51(3):264-283. 10.4132/jptm.2016.12.20.
Characteristic Changes in Decidual Gene Expression Signature in Spontaneous Term Parturition
- Affiliations
-
- 1Perinatology Research Branch, NICHD/NIH/DHHS, Bethesda, MD, and Detroit, MI, USA. than.gabor@ttk.mta.hu, prbchiefstaff@med.wayne.edu
- 2Department of Immunology, Eotvos Lorand University, Budapest, Hungary.
- 3Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor, MI, USA.
- 4Department of Epidemiology and Biostatistics, Michigan State University, East Lansing, MI, USA.
- 5Center for Molecular Medicine and Genetics, Wayne State University, Detroit, MI, USA.
- 6Department of Obstetrics and Gynecology, Wayne State University, School of Medicine, Detroit, MI, USA.
- 7Maternity Private Department, Kutvolgyi Clinical Block, Semmelweis University, Budapest, Hungary.
- 8Department of Pathology, Wayne State University, School of Medicine, Detroit, MI, USA.
- 9Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 10Systems Biology of Reproduction Lendulet Research Group, Institute of Enzymology, Research Centre for Natural Sciences, Hungarian Academy of Sciences, Budapest, Hungary.
- 11First Department of Pathology and Experimental Cancer Research, Semmelweis University, Budapest, Hungary.
- KMID: 2392594
- DOI: http://doi.org/10.4132/jptm.2016.12.20
Abstract
- BACKGROUND
The decidua has been implicated in the "terminal pathway" of human term parturition, which is characterized by the activation of pro-inflammatory pathways in gestational tissues. However, the transcriptomic changes in the decidua leading to terminal pathway activation have not been systematically explored. This study aimed to compare the decidual expression of developmental signaling and inflammation-related genes before and after spontaneous term labor in order to reveal their involvement in this process.
METHODS
Chorioamniotic membranes were obtained from normal pregnant women who delivered at term with spontaneous labor (TIL, n = 14) or without labor (TNL, n = 15). Decidual cells were isolated from snap-frozen chorioamniotic membranes with laser microdissection. The expression of 46 genes involved in decidual development, sex steroid and prostaglandin signaling, as well as pro- and anti-inflammatory pathways, was analyzed using high-throughput quantitative real-time polymerase chain reaction (qRT-PCR). Chorioamniotic membrane sections were immunostained and then semi-quantified for five proteins, and immunoassays for three chemokines were performed on maternal plasma samples.
RESULTS
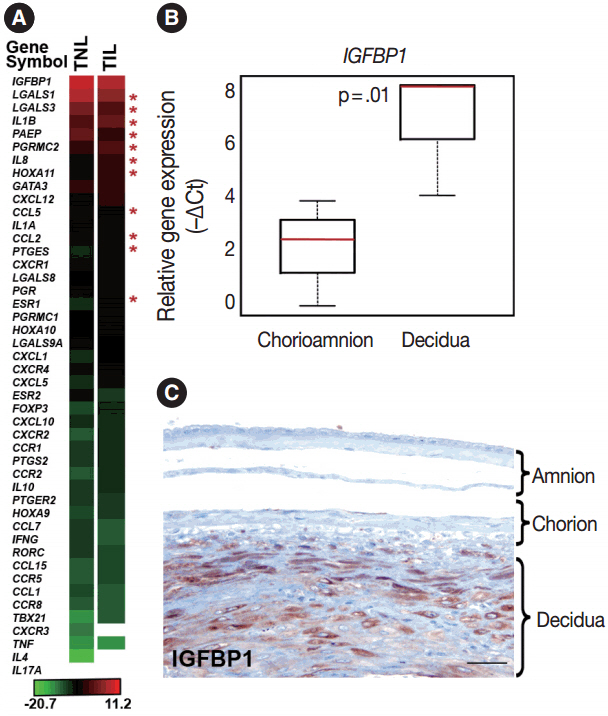
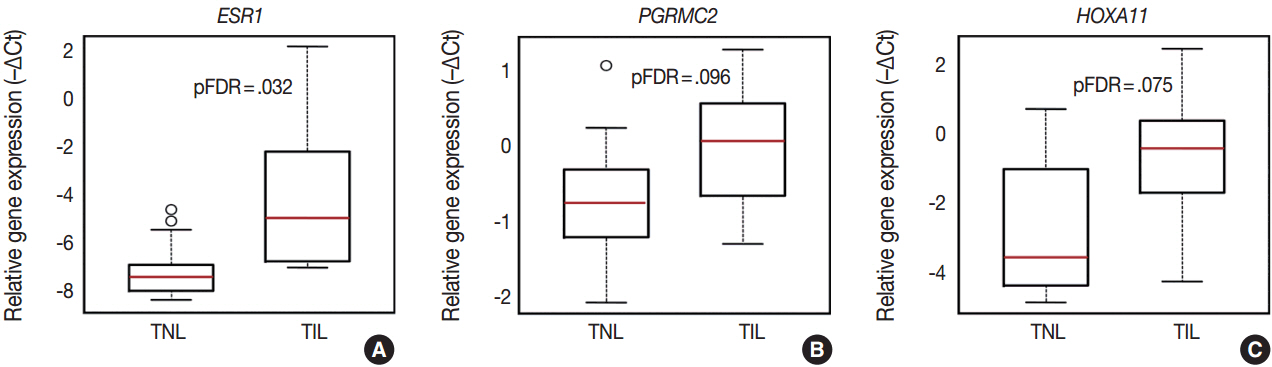
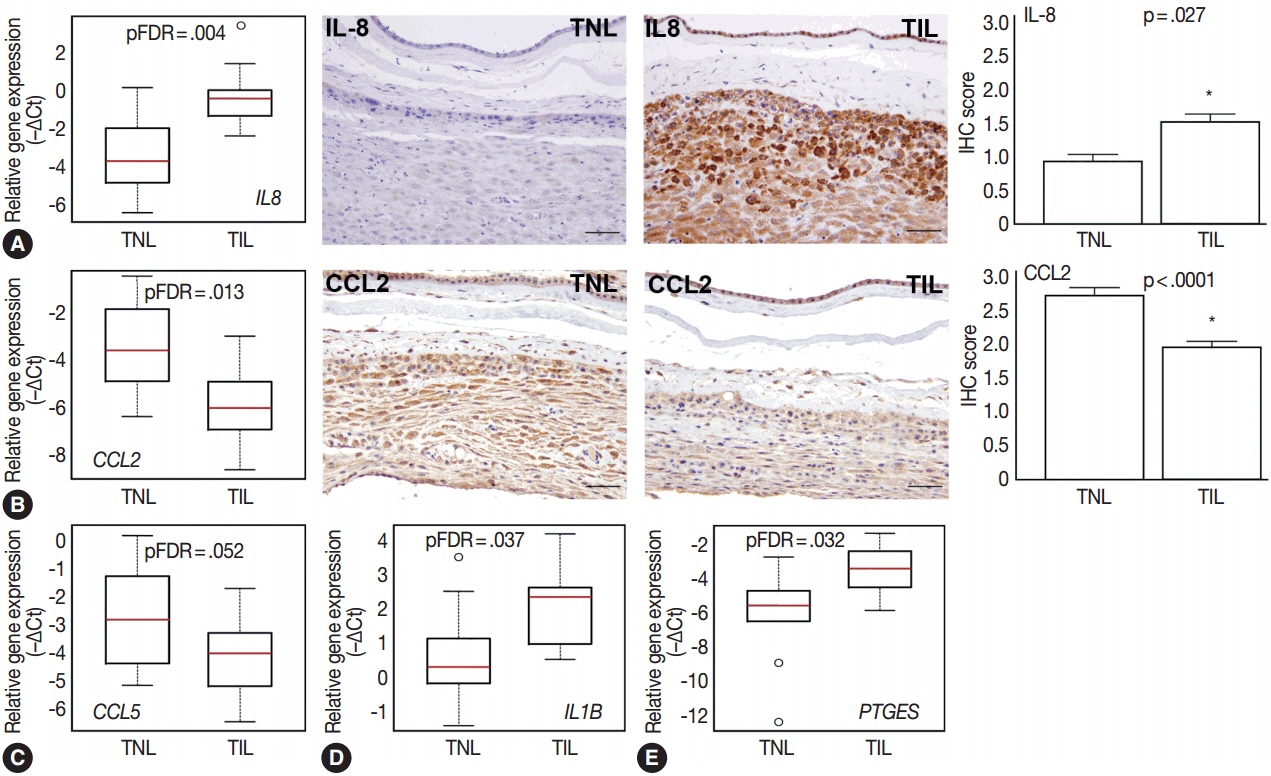
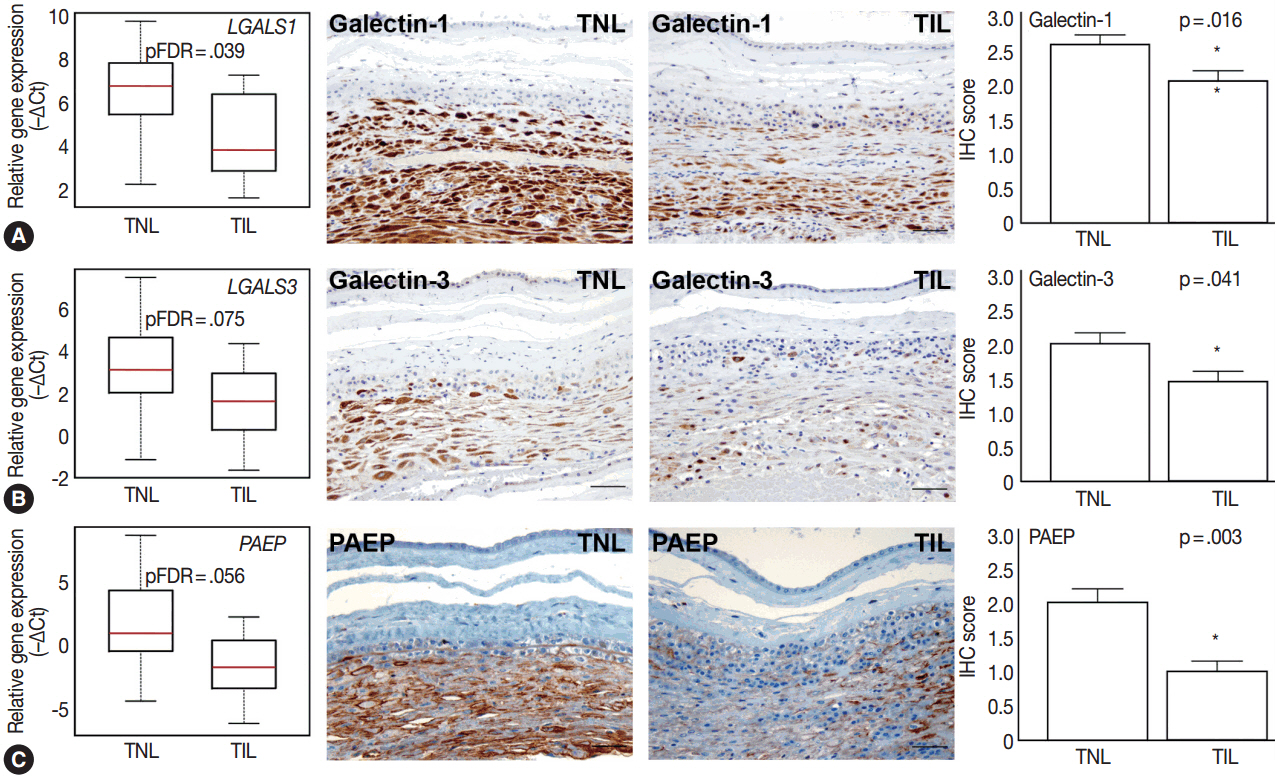
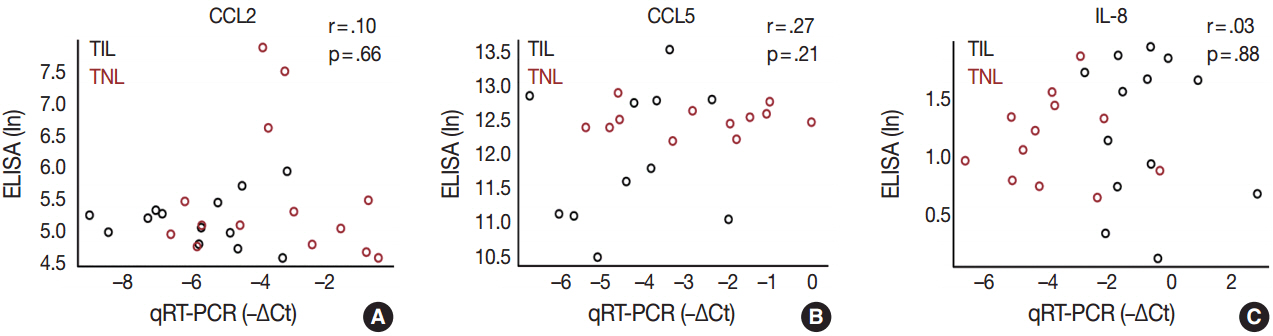
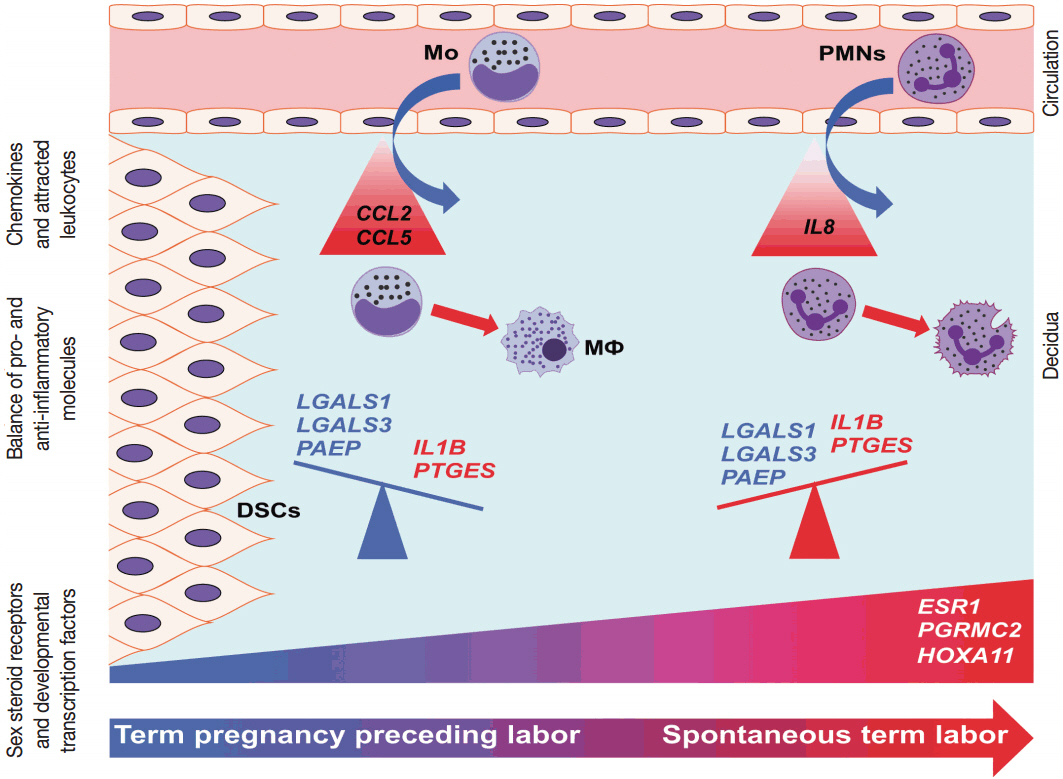
The genes with the highest expression in the decidua at term gestation included insulin-like growth factor-binding protein 1 (IGFBP1), galectin-1 (LGALS1), and progestogen-associated endometrial protein (PAEP); the expression of estrogen receptor 1 (ESR1), homeobox A11 (HOXA11), interleukin 1β (IL1B), IL8, progesterone receptor membrane component 2 (PGRMC2), and prostaglandin E synthase (PTGES) was higher in TIL than in TNL cases; the expression of chemokine C-C motif ligand 2 (CCL2), CCL5, LGALS1, LGALS3, and PAEP was lower in TIL than in TNL cases; immunostaining confirmed qRT-PCR data for IL-8, CCL2, galectin-1, galectin-3, and PAEP; and no correlations between the decidual gene expression and the maternal plasma protein concentrations of CCL2, CCL5, and IL-8 were found.
CONCLUSIONS
Our data suggests that with the initiation of parturition, the decidual expression of anti-inflammatory mediators decreases, while the expression of pro-inflammatory mediators and steroid receptors increases. This shift may affect downstream signaling pathways that can lead to parturition.
Keyword
MeSH Terms
-
Chemokines
Cytokines
Decidua
Estrogen Receptor alpha
Estrogens
Female
Galectin 1
Galectin 3
Galectins
Gene Expression*
Genes, Homeobox
Humans
Immunoassay
Interleukin-8
Interleukins
Leukocytes
Membranes
Microdissection
Parturition*
Plasma
Pregnancy
Pregnant Women
Progesterone
Real-Time Polymerase Chain Reaction
Receptors, Progesterone
Receptors, Steroid
Sexual Development
Transcriptome*
Chemokines
Cytokines
Estrogen Receptor alpha
Estrogens
Galectin 1
Galectin 3
Galectins
Interleukin-8
Interleukins
Progesterone
Receptors, Progesterone
Receptors, Steroid
Figure
Reference
-
1. Challis JR, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev. 2000; 21:514–50.
Article2. Iliodromiti Z, Antonakopoulos N, Sifakis S, et al. Endocrine, paracrine, and autocrine placental mediators in labor. Hormones (Athens). 2012; 11:397–409.
Article3. Zakar T, Hertelendy F. Progesterone withdrawal: key to parturition. Am J Obstet Gynecol. 2007; 196:289–96.
Article4. Pepe GJ, Albrecht ED. Actions of placental and fetal adrenal steroid hormones in primate pregnancy. Endocr Rev. 1995; 16:608–48.
Article5. Tan H, Yi L, Rote NS, Hurd WW, Mesiano S. Progesterone receptor-A and -B have opposite effects on proinflammatory gene expression in human myometrial cells: implications for progesterone actions in human pregnancy and parturition. J Clin Endocrinol Metab. 2012; 97:E719–30.
Article6. Csapo A. Progesterone block. Am J Anat. 1956; 98:273–91.
Article7. Mesiano S, Chan EC, Fitter JT, Kwek K, Yeo G, Smith R. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab. 2002; 87:2924–30.
Article8. Oh SY, Kim CJ, Park I, et al. Progesterone receptor isoform (A/B) ratio of human fetal membranes increases during term parturition. Am J Obstet Gynecol. 2005; 193(3 Pt 2):1156–60.
Article9. Mesiano S, Wang Y, Norwitz ER. Progesterone receptors in the human pregnancy uterus: do they hold the key to birth timing? Reprod Sci. 2011; 18:6–19.10. Bollapragada S, Youssef R, Jordan F, Greer I, Norman J, Nelson S. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am J Obstet Gynecol. 2009; 200:104.e1–11.11. Hassan SS, Romero R, Haddad R, et al. The transcriptome of the uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol. 2006; 195:778–86.
Article12. Stephen GL, Lui S, Hamilton SA, et al. Transcriptomic profiling of human choriodecidua during term labor: inflammation as a key driver of labor. Am J Reprod Immunol. 2015; 73:36–55.
Article13. Marvin KW, Keelan JA, Eykholt RL, Sato TA, Mitchell MD. Use of cDNA arrays to generate differential expression profiles for inflammatory genes in human gestational membranes delivered at term and preterm. Mol Hum Reprod. 2002; 8:399–408.
Article14. Chaemsaithong P, Madan I, Romero R, et al. Characterization of the myometrial transcriptome in women with an arrest of dilatation during labor. J Perinat Med. 2013; 41:665–81.
Article15. Thomson AJ, Telfer JF, Young A, et al. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999; 14:229–36.
Article16. Gomez-Lopez N, Tanaka S, Zaeem Z, Metz GA, Olson DM. Maternal circulating leukocytes display early chemotactic responsiveness during late gestation. BMC Pregnancy Childbirth. 2013; 13 Suppl 1:S8.
Article17. Sakamoto Y, Moran P, Bulmer JN, Searle RF, Robson SC. Macrophages and not granulocytes are involved in cervical ripening. J Reprod Immunol. 2005; 66:161–73.
Article18. Haddad R, Tromp G, Kuivaniemi H, et al. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol. 2006; 195:394.e1–24.
Article19. Gomez-Lopez N, Estrada-Gutierrez G, Jimenez-Zamudio L, VegaSanchez R, Vadillo-Ortega F. Fetal membranes exhibit selective leukocyte chemotaxic activity during human labor. J Reprod Immunol. 2009; 80:122–31.
Article20. Gomez-Lopez N, Laresgoiti-Servitje E, Olson DM, Estrada-Gutiérrez G, Vadillo-Ortega F. The role of chemokines in term and premature rupture of the fetal membranes: a review. Biol Reprod. 2010; 82:809–14.21. Gomez-Lopez N, Vadillo-Perez L, Nessim S, Olson DM, Vadillo-Ortega F. Choriodecidua and amnion exhibit selective leukocyte chemotaxis during term human labor. Am J Obstet Gynecol. 2011; 204:364.e9–16.
Article22. Osman I, Young A, Jordan F, Greer IA, Norman JE. Leukocyte density and proinflammatory mediator expression in regional human fetal membranes and decidua before and during labor at term. J Soc Gynecol Investig. 2006; 13:97–103.23. Gomez-Lopez N, Vadillo-Perez L, Hernandez-Carbajal A, Godines-Enriquez M, Olson DM, Vadillo-Ortega F. Specific inflammatory microenvironments in the zones of the fetal membranes at term delivery. Am J Obstet Gynecol. 2011; 205:235.e15–24.
Article24. Yellon SM, Mackler AM, Kirby MA. The role of leukocyte traffic and activation in parturition. J Soc Gynecol Investig. 2003; 10:323–38.
Article25. Hua R, Pease JE, Sooranna SR, et al. Stretch and inflammatory cytokines drive myometrial chemokine expression via NF-kappaB activation. Endocrinology. 2012; 153:481–91.26. Kayisli UA, Mahutte NG, Arici A. Uterine chemokines in reproductive physiology and pathology. Am J Reprod Immunol. 2002; 47:213–21.
Article27. Young A, Thomson AJ, Ledingham M, Jordan F, Greer IA, Norman JE. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol Reprod. 2002; 66:445–9.28. Montes MJ, Tortosa CG, Borja C, et al. Constitutive secretion of interleukin-6 by human decidual stromal cells in culture: regulatory effect of progesterone. Am J Reprod Immunol. 1995; 34:188–94.
Article29. Romero R, Mazor M, Brandt F, et al. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol. 1992; 27:117–23.30. Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, Williams J. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol. 1992; 166:1576–87.
Article31. Keelan JA, Marvin KW, Sato TA, Coleman M, McCowan LM, Mitchell MD. Cytokine abundance in placental tissues: evidence of inflammatory activation in gestational membranes with term and preterm parturition. Am J Obstet Gynecol. 1999; 181:1530–6.
Article32. Gomez-Lopez N, Hernandez-Santiago S, Lobb AP, Olson DM, Vadillo-Ortega F. Normal and premature rupture of fetal membranes at term delivery differ in regional chemotactic activity and related chemokine/cytokine production. Reprod Sci. 2013; 20:276–84.
Article33. Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory processes in preterm and term parturition. J Reprod Immunol. 2008; 79:50–7.
Article34. Winkler M, Rath W. The role of cytokines in the induction of labor, cervical ripening and rupture of the fetal membranes. Z Geburtshilfe Neonatol. 1996; 200 Suppl 1:1–12.35. Bowen JM, Chamley L, Keelan JA, Mitchell MD. Cytokines of the placenta and extra-placental membranes: roles and regulation during human pregnancy and parturition. Placenta. 2002; 23:257–73.
Article36. Mitchell MD, Dudley DJ, Edwin SS, Schiller SL. Interleukin-6 stimulates prostaglandin production by human amnion and decidual cells. Eur J Pharmacol. 1991; 192:189–91.
Article37. Belt AR, Baldassare JJ, Molnár M, Romero R, Hertelendy F. The nuclear transcription factor NF-kappaB mediates interleukin-1beta-induced expression of cyclooxygenase-2 in human myometrial cells. Am J Obstet Gynecol. 1999; 181:359–66.38. Kniss DA, Rovin B, Fertel RH, Zimmerman PD. Blockade NF-kappaB activation prohibits TNF-alpha-induced cyclooxygenase-2 gene expression in ED27 trophoblast-like cells. Placenta. 2001; 22:80–9.39. Ackerman WE 4th, Summerfield TL, Vandre DD, Robinson JM, Kniss DA. Nuclear factor-kappa B regulates inducible prostaglandin E synthase expression in human amnion mesenchymal cells. Biol Reprod. 2008; 78:68–76.40. Li R, Ackerman WE 4th, Summerfield TL, et al. Inflammatory gene regulatory networks in amnion cells following cytokine stimulation: translational systems approach to modeling human parturition. PLoS One. 2011; 6:e20560.
Article41. Mitchell BF, Taggart MJ. Are animal models relevant to key aspects of human parturition? Am J Physiol Regul Integr Comp Physiol. 2009; 297:R525–45.
Article42. Maymon E, Ghezzi F, Edwin SS, et al. The tumor necrosis factor alpha and its soluble receptor profile in term and preterm parturition. Am J Obstet Gynecol. 1999; 181(5 Pt 1):1142–8.43. Romero R, Sepulveda W, Mazor M, et al. The natural interleukin-1 receptor antagonist in term and preterm parturition. Am J Obstet Gynecol. 1992; 167(4 Pt 1):863–72.44. Ammala M, Nyman T, Salmi A, Rutanen EM. The interleukin-1 system in gestational tissues at term: effect of labour. Placenta. 1997; 18:717–23.
Article45. Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition: a review. Placenta. 2003; 24 Suppl A:S33–46.46. Dudley DJ, Hunter C, Mitchell MD, Varner MW. Amniotic fluid interleukin-10 (IL-10) concentrations during pregnancy and with labor. J Reprod Immunol. 1997; 33:147–56.
Article47. Jones CA, Finlay-Jones JJ, Hart PH. Type-1 and type-2 cytokines in human late-gestation decidual tissue. Biol Reprod. 1997; 57:303–11.48. Gotsch F, Romero R, Kusanovic JP, et al. The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/inflammation, and spontaneous parturition at term: a role for interleukin-10. J Matern Fetal Neonatal Med. 2008; 21:529–47.
Article49. Dudley DJ, Hunter C, Varner MW, Mitchell MD. Elevation of amniotic fluid interleukin-4 concentrations in women with preterm labor and chorioamnionitis. Am J Perinatol. 1996; 13:443–7.
Article50. Than NG, Romero R, Goodman M, et al. A primate subfamily of galectins expressed at the maternal-fetal interface that promote immune cell death. Proc Natl Acad Sci U S A. 2009; 106:9731–6.
Article51. Barrientos G, Freitag N, Tirado-Gonzalez I, et al. Involvement of galectin-1 in reproduction: past, present and future. Hum Reprod Update. 2014; 20:175–93.
Article52. Than NG, Romero R, Balogh A, et al. Galectins: Double-edged swords in the cross-roads of pregnancy complications and female reproductive tract inflammation and neoplasia. J Pathol Transl Med. 2015; 49:181–208.
Article53. Julkunen M, Rutanen EM, Koskimies A, Ranta T, Bohn H, Seppala M. Distribution of placental protein 14 in tissues and body fluids during pregnancy. Br J Obstet Gynaecol. 1985; 92:1145–51.
Article54. Phillips B, Knisley K, Weitlauf KD, Dorsett J, Lee V, Weitlauf H. Differential expression of two beta-galactoside-binding lectins in the reproductive tracts of pregnant mice. Biol Reprod. 1996; 55:548–58.55. Lockwood CJ, Paidas M, Krikun G, et al. Inflammatory cytokine and thrombin regulation of interleukin-8 and intercellular adhesion molecule-1 expression in first trimester human decidua. J Clin Endocrinol Metab. 2005; 90:4710–5.
Article56. Huang SJ, Schatz F, Masch R, et al. Regulation of chemokine production in response to pro-inflammatory cytokines in first trimester decidual cells. J Reprod Immunol. 2006; 72:60–73.
Article57. Sarno J, Schatz F, Huang SJ, Lockwood C, Taylor HS. Thrombin and interleukin-1beta decrease HOX gene expression in human first trimester decidual cells: implications for pregnancy loss. Mol Hum Reprod. 2009; 15:451–7.58. Segerer SE, Martignoni F, Bogdan A, et al. Thrombopoietin modulates the proliferation, migration and cytokine profile of decidual cell subsets during early gestation. Mol Hum Reprod. 2013; 19:361–8.
Article59. Kim JS, Romero R, Cushenberry E, et al. Distribution of CD14+ and CD68+ macrophages in the placental bed and basal plate of women with preeclampsia and preterm labor. Placenta. 2007; 28:571–6.
Article60. Kim SY, Romero R, Tarca AL, et al. Methylome of fetal and maternal monocytes and macrophages at the feto-maternal interface. Am J Reprod Immunol. 2012; 68:8–27.
Article61. Paavola LG, Furth EE, Delgado V, et al. Striking changes in the structure and organization of rat fetal membranes precede parturition. Biol Reprod. 1995; 53:321–38.62. Norwitz ER, Robinson JN, Challis JR. The control of labor. N Engl J Med. 1999; 341:660–6.
Article63. Mittal P, Romero R, Tarca AL, et al. A molecular signature of an arrest of descent in human parturition. Am J Obstet Gynecol. 2011; 204:177.e15–33.
Article64. Norwitz ER, Bonney EA, Snegovskikh VV, et al. Molecular regulation of parturition: the role of the decidual clock. Cold Spring Harb Perspect Med. 2015; 5:a023143.
Article65. Menon R, Bonney EA, Condon J, Mesiano S, Taylor RN. Novel concepts on pregnancy clocks and alarms: redundancy and synergy in human parturition. Hum Reprod Update. 2016; 22:535–60.
Article66. Daftary GS, Taylor HS. Implantation in the human: the role of HOX genes. Semin Reprod Med. 2000; 18:311–20.
Article67. Cermik D, Karaca M, Taylor HS. HOXA10 expression is repressed by progesterone in the myometrium: differential tissue-specific regulation of HOX gene expression in the reproductive tract. J Clin Endocrinol Metab. 2001; 86:3387–92.
Article68. Hsieh-Li HM, Witte DP, Weinstein M, et al. Hoxa 11 structure, extensive antisense transcription, and function in male and female fertility. Development. 1995; 121:1373–85.
Article69. Taylor HS. The role of HOX genes in the development and function of the female reproductive tract. Semin Reprod Med. 2000; 18:81–9.
Article70. Wong KH, Wintch HD, Capecchi MR. HOXA11 regulates stromal cell death and proliferation during neonatal uterine development. Mol Endocrinol. 2004; 18:184–93.
Article71. Lu Z, Hardt J, Kim JJ. Global analysis of genes regulated by HOXA10 in decidualization reveals a role in cell proliferation. Mol Hum Reprod. 2008; 14:357–66.
Article72. Lynch VJ, Tanzer A, Wang Y, et al. Adaptive changes in the transcription factor HoxA-11 are essential for the evolution of pregnancy in mammals. Proc Natl Acad Sci U S A. 2008; 105:14928–33.
Article73. Xu B, Geerts D, Bu Z, et al. Regulation of endometrial receptivity by the highly expressed HOXA9, HOXA11 and HOXD10 HOX-class homeobox genes. Hum Reprod. 2014; 29:781–90.74. Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996; 87:163–8.
Article75. Redline RW. Placental pathology: a systematic approach with clinical correlations. Placenta. 2008; 29 Suppl A:S86–91.
Article76. Kim CJ, Romero R, Kusanovic JP, et al. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod Pathol. 2010; 23:1000–11.
Article77. Than NG, Abdul Rahman O, Magenheim R, et al. Placental protein 13 (galectin-13) has decreased placental expression but increased shedding and maternal serum concentrations in patients presenting with preterm pre-eclampsia and HELLP syndrome. Virchows Arch. 2008; 453:387–400.
Article78. R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing;2011.79. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Methodol. 1995; 57:289–300.
Article80. Than NG, Romero R, Erez O, et al. Emergence of hormonal and redox regulation of galectin-1 in placental mammals: implication in maternal-fetal immune tolerance. Proc Natl Acad Sci U S A. 2008; 105:15819–24.
Article81. Han VK, Bassett N, Walton J, Challis JR. The expression of insulinlike growth factor (IGF) and IGF-binding protein (IGFBP) genes in the human placenta and membranes: evidence for IGF-IGFBP interactions at the feto-maternal interface. J Clin Endocrinol Metab. 1996; 81:2680–93.
Article82. Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG. 2006; 113 Suppl 3:17–42.
Article83. Romero R, Espinoza J, Gotsch F, et al. The use of high-dimensional biology (genomics, transcriptomics, proteomics, and metabolomics) to understand the preterm parturition syndrome. BJOG. 2006; 113 Suppl 3:118–35.
Article84. Eidem HR, Ackerman WE 4th, McGary KL, Abbot P, Rokas A. Gestational tissue transcriptomics in term and preterm human pregnancies: a systematic review and meta-analysis. BMC Med Genomics. 2015; 8:27.
Article85. Taylor HS, Arici A, Olive D, Igarashi P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest. 1998; 101:1379–84.
Article86. Taylor HS, Igarashi P, Olive DL, Arici A. Sex steroids mediate HOXA11 expression in the human peri-implantation endometrium. J Clin Endocrinol Metab. 1999; 84:1129–35.
Article87. Bisits AM, Smith R, Mesiano S, et al. Inflammatory aetiology of human myometrial activation tested using directed graphs. PLoS Comput Biol. 2005; 1:132–6.
Article88. Welsh T, Johnson M, Yi L, et al. Estrogen receptor (ER) expression and function in the pregnant human myometrium: estradiol via ERalpha activates ERK1/2 signaling in term myometrium. J Endocrinol. 2012; 212:227–38.89. Brosens JJ, Hayashi N, White JO. Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology. 1999; 140:4809–20.90. Tan J, Paria BC, Dey SK, Das SK. Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology. 1999; 140:5310–21.91. Gellersen B, Fernandes MS, Brosens JJ. Non-genomic progesterone actions in female reproduction. Hum Reprod Update. 2009; 15:119–38.
Article92. Karteris E, Zervou S, Pang Y, et al. Progesterone signaling in human myometrium through two novel membrane G protein-coupled receptors: potential role in functional progesterone withdrawal at term. Mol Endocrinol. 2006; 20:1519–34.
Article93. Thomas P, Pang Y, Dong J. Enhancement of cell surface expression and receptor functions of membrane progestin receptor alpha (mPRalpha) by progesterone receptor membrane component 1 (PGRMC1): evidence for a role of PGRMC1 as an adaptor protein for steroid receptors. Endocrinology. 2014; 155:1107–19.94. Shankar R, Johnson MP, Williamson NA, et al. Molecular markers of preterm labor in the choriodecidua. Reprod Sci. 2010; 17:297–310.
Article95. Salih SM, Taylor HS. HOXA10 gene expression in human fallopian tube and ectopic pregnancy. Am J Obstet Gynecol. 2004; 190:1404–6.
Article96. Evans KN, Bulmer JN, Kilby MD, Hewison M. Vitamin D and placental-decidual function. J Soc Gynecol Investig. 2004; 11:263–71.
Article97. Lynch VJ, Brayer K, Gellersen B, Wagner GP. HoxA-11 and FOXO1A cooperate to regulate decidual prolactin expression: towards inferring the core transcriptional regulators of decidual genes. PLoS One. 2009; 4:e6845.
Article98. Wagner GP, Kin K, Muglia L, Pavlicev M. Evolution of mammalian pregnancy and the origin of the decidual stromal cell. Int J Dev Biol. 2014; 58:117–26.
Article99. Lappas M. Forkhead box O1 (FOXO1) in pregnant human myometrial cells: a role as a pro-inflammatory mediator in human parturition. J Reprod Immunol. 2013; 99:24–32.
Article100. Dudley DJ, Edwin SS, Dangerfield A, Van Waggoner J, Mitchell MD. Regulation of cultured human chorion cell chemokine production by group B streptococci and purified bacterial products. Am J Reprod Immunol. 1996; 36:264–8.
Article101. Dudley DJ, Edwin SS, Van Wagoner J, Augustine NH, Hill HR, Mitchell MD. Regulation of decidual cell chemokine production by group B streptococci and purified bacterial cell wall components. Am J Obstet Gynecol. 1997; 177:666–72.
Article102. Mittal P, Romero R, Tarca AL, et al. Characterization of the myometrial transcriptome and biological pathways of spontaneous human labor at term. J Perinat Med. 2010; 38:617–43.
Article103. Tornblom SA, Klimaviciute A, Byström B, Chromek M, Brauner A, Ekman-Ordeberg G. Non-infected preterm parturition is related to increased concentrations of IL-6, IL-8 and MCP-1 in human cervix. Reprod Biol Endocrinol. 2005; 3:39.
Article104. Shynlova O, Tsui P, Dorogin A, Lye SJ. Monocyte chemoattractant protein-1 (CCL-2) integrates mechanical and endocrine signals that mediate term and preterm labor. J Immunol. 2008; 181:1470–9.
Article105. Esplin MS, Peltier MR, Hamblin S, et al. Monocyte chemotactic protein-1 expression is increased in human gestational tissues during term and preterm labor. Placenta. 2005; 26:661–71.
Article106. Jones RL, Hannan NJ, Kaitu’u TJ, Zhang J, Salamonsen LA. Identification of chemokines important for leukocyte recruitment to the human endometrium at the times of embryo implantation and menstruation. J Clin Endocrinol Metab. 2004; 89:6155–67.
Article107. Engert S, Rieger L, Kapp M, Becker JC, Dietl J, Kämmerer U. Profiling chemokines, cytokines and growth factors in human early pregnancy decidua by protein array. Am J Reprod Immunol. 2007; 58:129–37.
Article108. Li M, Wu ZM, Yang H, Huang SJ. NFkappaB and JNK/MAPK activation mediates the production of major macrophage- or dendritic cell-recruiting chemokine in human first trimester decidual cells in response to proinflammatory stimuli. J Clin Endocrinol Metab. 2011; 96:2502–11.109. Athayde N, Romero R, Maymon E, et al. A role for the novel cytokine RANTES in pregnancy and parturition. Am J Obstet Gynecol. 1999; 181:989–94.
Article110. Mackler AM, Iezza G, Akin MR, McMillan P, Yellon SM. Macrophage trafficking in the uterus and cervix precedes parturition in the mouse. Biol Reprod. 1999; 61:879–83.
Article111. Shynlova O, Nedd-Roderique T, Li Y, Dorogin A, Nguyen T, Lye SJ. Infiltration of myeloid cells into decidua is a critical early event in the labour cascade and post-partum uterine remodelling. J Cell Mol Med. 2013; 17:311–24.
Article112. Hamilton S, Oomomian Y, Stephen G, et al. Macrophages infiltrate the human and rat decidua during term and preterm labor: evidence that decidual inflammation precedes labor. Biol Reprod. 2012; 86:39.
Article113. Srikhajon K, Shynlova O, Preechapornprasert A, Chanrachakul B, Lye S. A new role for monocytes in modulating myometrial inflammation during human labor. Biol Reprod. 2014; 91:10.
Article114. Gomez-Lopez N, Vega-Sanchez R, Castillo-Castrejon M, Romero R, Cubeiro-Arreola K, Vadillo-Ortega F. Evidence for a role for the adaptive immune response in human term parturition. Am J Reprod Immunol. 2013; 69:212–30.
Article115. Xu Y, Romero R, Miller D, et al. An M1-like macrophage polarization in decidual tissue during spontaneous preterm labor that is attenuated by rosiglitazone treatment. J Immunol. 2016; 196:2476–91.
Article116. Hamilton SA, Tower CL, Jones RL. Identification of chemokines associated with the recruitment of decidual leukocytes in human labour: potential novel targets for preterm labour. PLoS One. 2013; 8:e56946.
Article117. Peveri P, Walz A, Dewald B, Baggiolini M. A novel neutrophil-activating factor produced by human mononuclear phagocytes. J Exp Med. 1988; 167:1547–59.
Article118. Kelly RW, Illingworth P, Baldie G, Leask R, Brouwer S, Calder AA. Progesterone control of interleukin-8 production in endometrium and chorio-decidual cells underlines the role of the neutrophil in menstruation and parturition. Hum Reprod. 1994; 9:253–8.
Article119. Gervasi MT, Chaiworapongsa T, Pacora P, et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am J Obstet Gynecol. 2001; 185:792–7.
Article120. Yuan M, Jordan F, McInnes IB, Harnett MM, Norman JE. Leukocytes are primed in peripheral blood for activation during term and preterm labour. Mol Hum Reprod. 2009; 15:713–24.
Article121. Gomez-Lopez N, StLouis D, Lehr MA, Sanchez-Rodriguez EN, Arenas-Hernandez M. Immune cells in term and preterm labor. Cell Mol Immunol. 2014; 11:571–81.
Article122. Giaglis S, Stoikou M, Grimolizzi F, et al. Neutrophil migration into the placenta: good, bad or deadly? Cell Adh Migr. 2016; 10:208–25.
Article123. Castillo-Castrejon M, Meraz-Cruz N, Gomez-Lopez N, et al. Choriodecidual cells from term human pregnancies show distinctive functional properties related to the induction of labor. Am J Reprod Immunol. 2014; 71:86–93.
Article124. Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol. 1991; 165(4 Pt 1):813–20.125. Tashima LS, Millar LK, Bryant-Greenwood GD. Genes upregulated in human fetal membranes by infection or labor. Obstet Gynecol. 1999; 94:441–9.
Article126. Osman I, Young A, Ledingham MA, et al. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod. 2003; 9:41–5.
Article127. Osmers RG, Bläser J, Kuhn W, Tschesche H. Interleukin-8 synthesis and the onset of labor. Obstet Gynecol. 1995; 86:223–9.
Article128. Sennström MK, Brauner A, Lu Y, Granström LM, Malmström AL, Ekman GE. Interleukin-8 is a mediator of the final cervical ripening in humans. Eur J Obstet Gynecol Reprod Biol. 1997; 74:89–92.
Article129. Sennström MB, Ekman G, Westergren-Thorsson G, et al. Human cervical ripening, an inflammatory process mediated by cytokines. Mol Hum Reprod. 2000; 6:375–81.130. Charpigny G, Leroy MJ, Breuiller-Fouché M, et al. A functional genomic study to identify differential gene expression in the preterm and term human myometrium. Biol Reprod. 2003; 68:2289–96.131. Bethin KE, Nagai Y, Sladek R, et al. Microarray analysis of uterine gene expression in mouse and human pregnancy. Mol Endocrinol. 2003; 17:1454–69.
Article132. Rehman KS, Yin S, Mayhew BA, Word RA, Rainey WE. Human myometrial adaptation to pregnancy: cDNA microarray gene expression profiling of myometrium from non-pregnant and pregnant women. Mol Hum Reprod. 2003; 9:681–700.
Article133. Axemo P, Brauner A, Pettersson M, Eriksson L, Rwamushaija E, Bergstrom S. Amniotic fluid interleukins in Swedish and Mozambican pregnant women. Gynecol Obstet Invest. 1996; 41:113–7.
Article134. Laham N, Rice GE, Bishop GJ, Ransome C, Brennecke SP. Interleukin 8 concentrations in amniotic fluid and peripheral venous plasma during human pregnancy and parturition. Acta Endocrinol (Copenh). 1993; 129:220–4.
Article135. Romero R, Brody DT, Oyarzun E, et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989; 160(5 Pt 1):1117–23.
Article136. Romero R, Wu YK, Brody DT, Oyarzun E, Duff GW, Durum SK. Human decidua: a source of interleukin-1. Obstet Gynecol. 1989; 73:31–4.137. Steinborn A, Geisse M, Kaufmann M. Expression of cytokine receptors in the placenta in term and preterm labour. Placenta. 1998; 19:165–70.
Article138. Romero R, Parvizi ST, Oyarzun E, et al. Amniotic fluid interleukin-1 in spontaneous labor at term. J Reprod Med. 1990; 35:235–8.139. Ibrahim SA, Ackerman WE 4th, Summerfield TL, Lockwood CJ, Schatz F, Kniss DA. Inflammatory gene networks in term human decidual cells define a potential signature for cytokine-mediated parturition. Am J Obstet Gynecol. 2016; 214:284.e1–47.
Article140. Dudley DJ, Trautman MS, Mitchell MD. Inflammatory mediators regulate interleukin-8 production by cultured gestational tissues: evidence for a cytokine network at the chorio-decidual interface. J Clin Endocrinol Metab. 1993; 76:404–10.
Article141. Alnaif B, Benzie RJ, Gibb W. Studies on the action of interleukin-1 on term human fetal membranes and decidua. Can J Physiol Pharmacol. 1994; 72:133–9.
Article142. Challis JR, Lye SJ. Parturition. Oxf Rev Reprod Biol. 1986; 8:61–129.143. Challis JR, Lye SJ, Gibb W. Prostaglandins and parturition. Ann N Y Acad Sci. 1997; 828:254–67.
Article144. Olson DM. The role of prostaglandins in the initiation of parturition. Best Pract Res Clin Obstet Gynaecol. 2003; 17:717–30.
Article145. Mitchell MD, Edwin S, Romero RJ. Prostaglandin biosynthesis by human decidual cells: effects of inflammatory mediators. Prostaglandins Leukot Essent Fatty Acids. 1990; 41:35–8.
Article146. Skinner KA, Challis JR. Changes in the synthesis and metabolism of prostaglandins by human fetal membranes and decidua at labor. Am J Obstet Gynecol. 1985; 151:519–23.
Article147. Fuentes A, Spaziani EP, O’Brien WF. The expression of cyclooxygenase-2 (COX-2) in amnion and decidua following spontaneous labor. Prostaglandins. 1996; 52:261–7.
Article148. Phillips RJ, Fortier MA, López Bernal A. Prostaglandin pathway gene expression in human placenta, amnion and choriodecidua is differentially affected by preterm and term labour and by uterine inflammation. BMC Pregnancy Childbirth. 2014; 14:241.
Article149. Phillips RJ, Al-Zamil H, Hunt LP, Fortier MA, López Bernal A. Genes for prostaglandin synthesis, transport and inactivation are differentially expressed in human uterine tissues, and the prostaglandin F synthase AKR1B1 is induced in myometrial cells by inflammatory cytokines. Mol Hum Reprod. 2011; 17:1–13.
Article150. Frasor J, Weaver AE, Pradhan M, Mehta K. Synergistic up-regulation of prostaglandin E synthase expression in breast cancer cells by 17beta-estradiol and proinflammatory cytokines. Endocrinology. 2008; 149:6272–9.151. Vargas ML, Sántos JL, Ruiz C, et al. Comparison of the proportions of leukocytes in early and term human decidua. Am J Reprod Immunol. 1993; 29:135–40.
Article152. Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004; 5:266–71.
Article153. Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004; 112:38–43.
Article154. Gomez-Lopez N, Guilbert LJ, Olson DM. Invasion of the leukocytes into the fetal-maternal interface during pregnancy. J Leukoc Biol. 2010; 88:625–33.
Article155. Mjösberg J, Berg G, Jenmalm MC, Ernerudh J. FOXP3+ regulatory T cells and T helper 1, T helper 2, and T helper 17 cells in human early pregnancy decidua. Biol Reprod. 2010; 82:698–705.156. Sindram-Trujillo AP, Scherjon SA, van Hulst-van Miert PP, Kanhai HH, Roelen DL, Claas FH. Comparison of decidual leukocytes following spontaneous vaginal delivery and elective cesarean section in uncomplicated human term pregnancy. J Reprod Immunol. 2004; 62:125–37.
Article157. Hughes RC. Galectins as modulators of cell adhesion. Biochimie. 2001; 83:667–76.
Article158. Rabinovich GA, Toscano MA. Turning ‘sweet’ on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat Rev Immunol. 2009; 9:338–52.
Article159. Hirota Y, Burnum KE, Acar N, Rabinovich GA, Daikoku T, Dey SK. Galectin-1 markedly reduces the incidence of resorptions in mice missing immunophilin FKBP52. Endocrinology. 2012; 153:2486–93.
Article160. Than NG, Romero R, Kim CJ, McGowen MR, Papp Z, Wildman DE. Galectins: guardians of eutherian pregnancy at the maternalfetal interface. Trends Endocrinol Metab. 2012; 23:23–31.
Article161. Szekeres-Bartho J, Halasz M, Palkovics T. Progesterone in pregnancy; receptor-ligand interaction and signaling pathways. J Reprod Immunol. 2009; 83:60–4.
Article162. von Wolff M, Wang X, Gabius HJ, Strowitzki T. Galectin fingerprinting in human endometrium and decidua during the menstrual cycle and in early gestation. Mol Hum Reprod. 2005; 11:189–94.
Article163. Tirado-González I, Freitag N, Barrientos G, et al. Galectin-1 influences trophoblast immune evasion and emerges as a predictive factor for the outcome of pregnancy. Mol Hum Reprod. 2013; 19:43–53.164. Blois SM, Ilarregui JM, Tometten M, et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med. 2007; 13:1450–7.
Article165. Than NG, Kim SS, Abbas A, et al. Chorioamnionitis and increased galectin-1 expression in PPROM: an anti-inflammatory response in the fetal membranes? Am J Reprod Immunol. 2008; 60:298–311.166. Dumic J, Dabelic S, Flögel M. Galectin-3: an open-ended story. Biochim Biophys Acta. 2006; 1760:616–35.
Article167. Seppälä M, Taylor RN, Koistinen H, Koistinen R, Milgrom E. Glycodelin: a major lipocalin protein of the reproductive axis with diverse actions in cell recognition and differentiation. Endocr Rev. 2002; 23:401–30.168. Than GN, Bohn H, Szabo DG. Advances in pregnancy-related protein research: functional and clinical applications. Boca Raton: CRC Press Inc.;1993.169. Alok A, Karande AA. The role of glycodelin as an immune-modulating agent at the feto-maternal interface. J Reprod Immunol. 2009; 83:124–7.
Article170. Taylor RN, Savouret JF, Vaisse C, et al. Promegestone (R5020) and mifepristone (RU486) both function as progestational agonists of human glycodelin gene expression in isolated human epithelial cells. J Clin Endocrinol Metab. 1998; 83:4006–12.
Article171. Li TC, Dalton C, Bolton AE, Ling E, Warren A, Cooke ID. An analysis of the variation of plasma concentrations of placental protein 14 in artificial cycles. Fertil Steril. 1992; 57:776–82.
Article172. Bolton AE, Pockley AG, Clough KJ, et al. Identification of placental protein 14 as an immunosuppressive factor in human reproduction. Lancet. 1987; 1:593–5.
Article173. Mukhopadhyay D, Sundereshan S, Rao C, Karande AA. Placental protein 14 induces apoptosis in T cells but not in monocytes. J Biol Chem. 2001; 276:28268–73.
Article174. Vigne JL, Hornung D, Mueller MD, Taylor RN. Purification and characterization of an immunomodulatory endometrial protein, glycodelin. J Biol Chem. 2001; 276:17101–5.
Article175. Alok A, Mukhopadhyay D, Karande AA. Glycodelin A, an immunomodulatory protein in the endometrium, inhibits proliferation and induces apoptosis in monocytic cells. Int J Biochem Cell Biol. 2009; 41:1138–47.
Article176. Simpson KL, Keelan JA, Mitchell MD. Labor-associated changes in interleukin-10 production and its regulation by immunomodulators in human choriodecidua. J Clin Endocrinol Metab. 1998; 83:4332–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Signature of arylacetamide deacetylase expression is associated with prognosis and immune infiltration in ovarian cancer
- The Production of Prostaglandin , Thromboxane and Cytokines in Human Term Decidual Cell Fraction by NG-nitro-L-arginine methyl ester Stimulation
- Long Noncoding RNA Signature and Disease Outcome in Estrogen Receptor-Positive Breast Cancer Patients Treated with Tamoxifen
- Tumor Necrosis Factor-alpha Gene Signature Is Absent in Peripheral Blood Mononuclear Cells of Patients with Granulomatosis Polyangiitis
- A case of term delivery in a woman with didelphic uterus after expelling the decidual cast at midtrimester: Case report