Allergen-Dependent Differences in ILC2s Frequencies in Patients With Allergic Rhinitis
- Affiliations
-
- 1Department of Otolaryngology Head and Neck Surgery and Department of Allergy, Beijing TongRen Hospital, Capital Medical University, Beijing 100730, China. dr.luozhang@139.com
- 2Beijing Key Laboratory of Nasal diseases, Beijing Institute of Otolaryngology, Beijing 100005, China.
- 3Upper Airways Research Laboratory, Department of Oto-Rhino-Laryngology, Ghent University Hospital, De Pintelaan 185, 9000 Ghent, Belgium.
- 4Department of Otorhinolaryngology Head and Neck Surgery, the First Affiliated Hospital, Harbin Medical University, Harbin 150001, Heilongjiang Province, China.
- KMID: 2391041
- DOI: http://doi.org/10.4168/aair.2016.8.3.216
Abstract
- PURPOSE
Group 2 innate lymphoid cells (ILC2s) are a novel population of lineage-negative cells that induce innate type 2 responses by producing the critical Th2-type cytokines IL-5 and IL-13 in response to IL-25 and IL-33 stimulation. ILC2s accumulation in the peripheral blood of patients with allergic rhinitis (AR) is controversial; the precise role of ILC2s in the immunopathogenesis of AR is still not clear. We investigated the role of ILC2s in phenotypic AR sensitized to distinct allergens.
METHODS
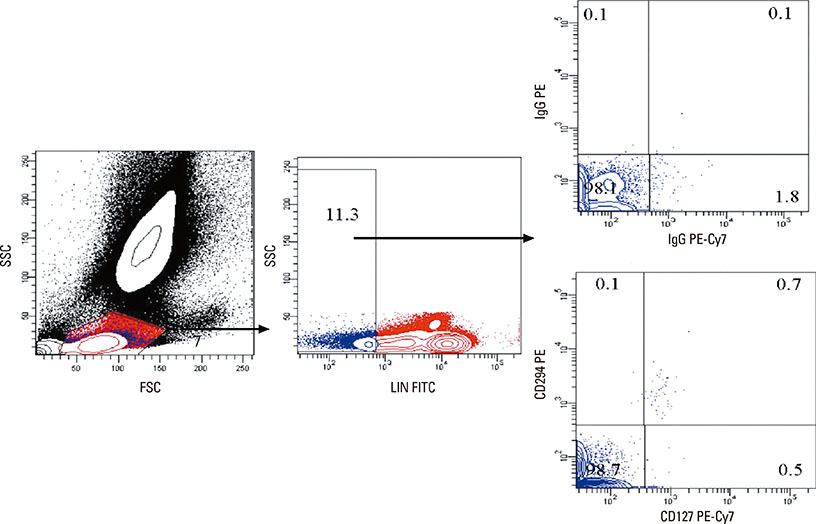
Flow cytometric analysis of the peripheral blood of 7 healthy controls (HCs), 9 patients monosensitized to house dust mite (HDM), and 8 patients monosensitized to mugwort was performed to quantify ILC2s frequency. Peripheral blood mononuclear cells (PBMCs) were isolated from HDM-AR and mugwort-AR patients, and Lineage- and Lineage+ cells were separated using a fluorescence-activated cell sorter (FACS). IL-5 and IL-13 levels in the supernatants of PBMCs, and Lineage- and Lineage+ cells stimulated with IL-25 and/or IL-33 combined with IL-2 in vitro were assessed using the Milliplex magnetic bead kit.
RESULTS
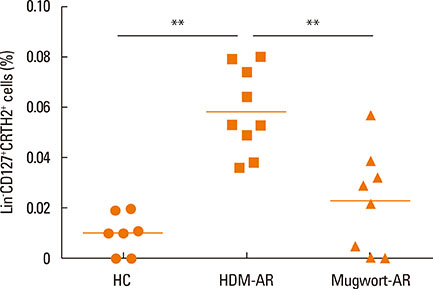
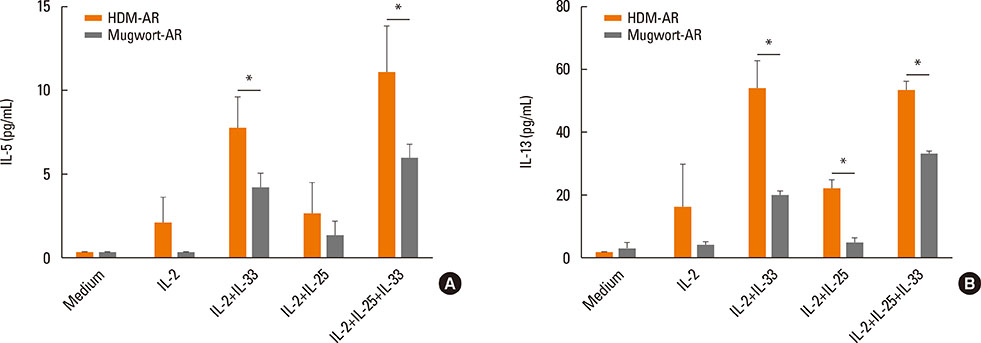
The percentage of ILC2s was significantly elevated in HDM-AR patients compared to mugwort-AR patients and HCs, while no significant difference was found between mugwort-AR patients and HCs. IL-33+/-IL-25 plus IL-2 induced a significantly greater release of IL-5 and IL-13 in the PBMCs of HDM-AR patients compared to PBMCs of mugwort-AR patients. IL-25 plus IL-2 also induced a significantly greater release of IL-13 in the PBMCs of HDM-AR patients compared to PBMCs of mugwort-AR patients. Stimulation with IL-33 and/or IL-25 combined with IL-2 also induced a significantly greater IL-5 and IL-13 release from Lineage- cells compared to Lineage+ cells.
CONCLUSIONS
AR patients sensitized to HDM or mugwort allergen have distinct phenotypic and functional profiles in ILC2s frequencies. ILC2s mediate major type 2 immunity in the development of HDM-AR and may be a potential therapeutic target.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Decreased CRTH2 Expression and Response to Allergen Re-stimulation on Innate Lymphoid Cells in Patients With Allergen-Specific Immunotherapy
Wat Mitthamsiri, Panitan Pradubpongsa, Atik Sangasapaviliya, Tadech Boonpiyathad
Allergy Asthma Immunol Res. 2018;10(6):662-674. doi: 10.4168/aair.2018.10.6.662.Chinese Society of Allergy Guidelines for Diagnosis and Treatment of Allergic Rhinitis
Lei Cheng, Jianjun Chen, Qingling Fu, Shaoheng He, Huabin Li, Zheng Liu, Guolin Tan, Zezhang Tao, Dehui Wang, Weiping Wen, Rui Xu, Yu Xu, Qintai Yang, Chonghua Zhang, Gehua Zhang, Ruxin Zhang, Yuan Zhang, Bing Zhou, Dongdong Zhu, Luquan Chen, Xinyan Cui, Yuqin Deng, Zhiqiang Guo, Zhenxiao Huang, Zizhen Huang, Houyong Li, Jingyun Li, Wenting Li, Yanqing Li, Lin Xi, Hongfei Lou, Meiping Lu, Yuhui Ouyang, Wendan Shi, Xiaoyao Tao, Huiqin Tian, Chengshuo Wang, Min Wang, Nan Wang, Xiangdong Wang, Hui Xie, Shaoqing Yu, Renwu Zhao, Ming Zheng, Han Zhou, Luping Zhu, Luo Zhang
Allergy Asthma Immunol Res. 2018;10(4):300-353. doi: 10.4168/aair.2018.10.4.300.
Reference
-
1. Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010; 463:540–544.2. Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010; 464:1367–1370.3. Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010; 107:11489–11494.4. Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006; 203:1105–1116.5. Kim HY, Chang YJ, Subramanian S, Lee HH, Albacker LA, Matangkasombut P, et al. Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. J Allergy Clin Immunol. 2012; 129:216–227.e1-6.6. Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011; 12:631–638.7. Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011; 12:1045–1054.8. Klein Wolterink RG, Kleinjan A, van Nimwegen M, Bergen I, de Bruijn M, Levani Y, et al. Pulmonary innate lymphoid cells are major producers of IL-5 and IL-13 in murine models of allergic asthma. Eur J Immunol. 2012; 42:1106–1116.9. Bartemes KR, Kephart GM, Fox SJ, Kita H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol. 2014; 134:671–678.e4.10. Meltzer EO, Szwarcberg J, Pill MW. Allergic rhinitis, asthma, and rhinosinusitis: diseases of the integrated airway. J Manag Care Pharm. 2004; 10:310–317.11. Pawankar R, Mori S, Ozu C, Kimura S. Overview on the pathomechanisms of allergic rhinitis. Asia Pac Allergy. 2011; 1:157–167.12. Mandhane SN, Shah JH, Thennati R. Allergic rhinitis: an update on disease, present treatments and future prospects. Int Immunopharmacol. 2011; 11:1646–1662.13. Doherty TA, Scott D, Walford HH, Khorram N, Lund S, Baum R, et al. Allergen challenge in allergic rhinitis rapidly induces increased peripheral blood type 2 innate lymphoid cells that express CD84. J Allergy Clin Immunol. 2014; 133:1203–1205.14. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008; 63:Suppl 86. 8–160.15. Mjösberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011; 12:1055–1062.16. Liao W, Lin JX, Leonard WJ. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr Opin Immunol. 2011; 23:598–604.17. Lao-Araya M, Steveling E, Scadding GW, Durham SR, Shamji MH. Seasonal increases in peripheral innate lymphoid type 2 cells are inhibited by subcutaneous grass pollen immunotherapy. J Allergy Clin Immunol. 2014; 134:1193–1195.e4.18. Himly M, Jahn-Schmid B, Dedic A, Kelemen P, Wopfner N, Altmann F, et al. Art v 1, the major allergen of mugwort pollen, is a modular glycoprotein with a defensin-like and a hydroxyproline-rich domain. FASEB J. 2003; 17:106–108.19. Jahn-Schmid B, Kelemen P, Himly M, Bohle B, Fischer G, Ferreira F, et al. The T cell response to Art v 1, the major mugwort pollen allergen, is dominated by one epitope. J Immunol. 2002; 169:6005–6011.20. Wan H, Winton HL, Soeller C, Tovey ER, Gruenert DC, Thompson PJ, et al. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest. 1999; 104:123–133.21. Herbert CA, King CM, Ring PC, Holgate ST, Stewart GA, Thompson PJ, et al. Augmentation of permeability in the bronchial epithelium by the house dust mite allergen Der p1. Am J Respir Cell Mol Biol. 1995; 12:369–378.22. Stewart GA, Ward LD, Simpson RJ, Thompson PJ. The group III allergen from the house dust mite Dermatophagoides pteronyssinus is a trypsin-like enzyme. Immunology. 1992; 75:29–35.23. Yasueda H, Mita H, Akiyama K, Shida T, Ando T, Sugiyama S, et al. Allergens from Dermatophagoides mites with chymotryptic activity. Clin Exp Allergy. 1993; 23:384–390.24. Asaka D, Yoshikawa M, Nakayama T, Yoshimura T, Moriyama H, Otori N. Elevated levels of interleukin-33 in the nasal secretions of patients with allergic rhinitis. Int Arch Allergy Immunol. 2012; 158:Suppl 1. 47–50.25. Chu DK, Llop-Guevara A, Walker TD, Flader K, Goncharova S, Boudreau JE, et al. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J Allergy Clin Immunol. 2013; 131:187–200.e1-8.26. Xue L, Salimi M, Panse I, Mjösberg JM, McKenzie AN, Spits H, et al. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J Allergy Clin Immunol. 2014; 133:1184–1194.27. Barlow JL, Peel S, Fox J, Panova V, Hardman CS, Camelo A, et al. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J Allergy Clin Immunol. 2013; 132:933–941.28. Shaw JL, Fakhri S, Citardi MJ, Porter PC, Corry DB, Kheradmand F, et al. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2013; 188:432–439.29. Miljkovic D, Bassiouni A, Cooksley C, Ou J, Hauben E, Wormald PJ, et al. Association between group 2 innate lymphoid cells enrichment, nasal polyps and allergy in chronic rhinosinusitis. Allergy. 2014; 69:1154–1161.30. Walford HH, Lund SJ, Baum RE, White AA, Bergeron CM, Husseman J, et al. Increased ILC2s in the eosinophilic nasal polyp endotype are associated with corticosteroid responsiveness. Clin Immunol. 2014; 155:126–135.31. Ho J, Bailey M, Zaunders J, Mrad N, Sacks R, Sewell W, et al. Group 2 innate lymphoid cells (ILC2s) are increased in chronic rhinosinusitis with nasal polyps or eosinophilia. Clin Exp Allergy. 2015; 45:394–403.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosis of Allergic Rhinitis
- Decreased CRTH2 Expression and Response to Allergen Re-stimulation on Innate Lymphoid Cells in Patients With Allergen-Specific Immunotherapy
- Current Update of Local Allergic Rhinitis
- Clinical Practice Guideline for Physicians on Allergic Rhinitis
- Allergen Specific Immunotherapy for Allergic Rhinitis