Allergy Asthma Immunol Res.
2018 Nov;10(6):662-674. 10.4168/aair.2018.10.6.662.
Decreased CRTH2 Expression and Response to Allergen Re-stimulation on Innate Lymphoid Cells in Patients With Allergen-Specific Immunotherapy
- Affiliations
-
- 1Division of Allergy and Clinical Immunology, Department of Medicine, Phramongkutklao Hospital, Bangkok, Thailand. tadechb@pmk.ac.th
- KMID: 2441815
- DOI: http://doi.org/10.4168/aair.2018.10.6.662
Abstract
- PURPOSE
Group 2 innate lymphoid cells (ILC2s) have been implicated in the pathogenesis of allergic disease. However, the effect of allergen-specific immunotherapy (AIT) on ILCs remains to be clarified. The aim of this study was to evaluate the levels of ILC subsets in allergic rhinitis (AR) patients in response to house dust mite (HDM)-specific immunotherapy.
METHODS
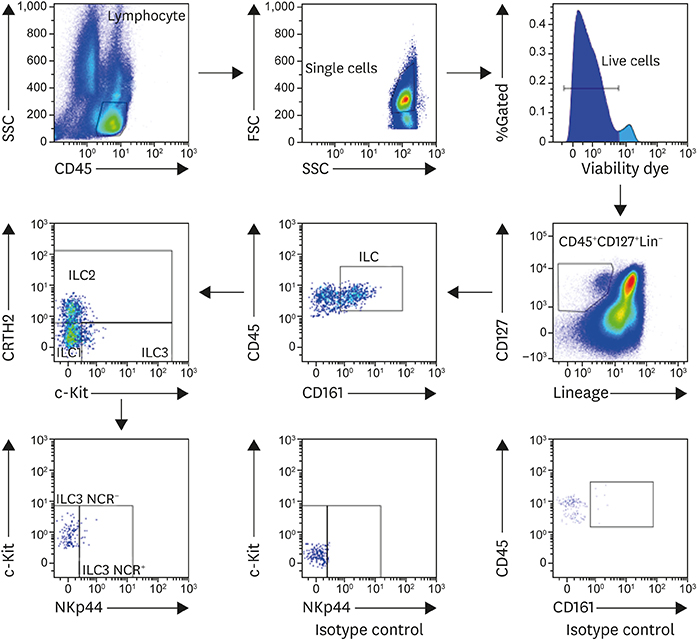
We enrolled 37 AR patients undergoing AIT (16 responders and 11 non-responders) for 2 years, 35 HDM AR patients and 28 healthy subjects. Peripheral blood mononuclear cells (PBMCs) were analyzed by flow cytometry to identify ILC subsets. Stimulation of ILC2s with recombinant allergen-specific protein was used to determine ILC2's activation (CD69 expression).
RESULTS
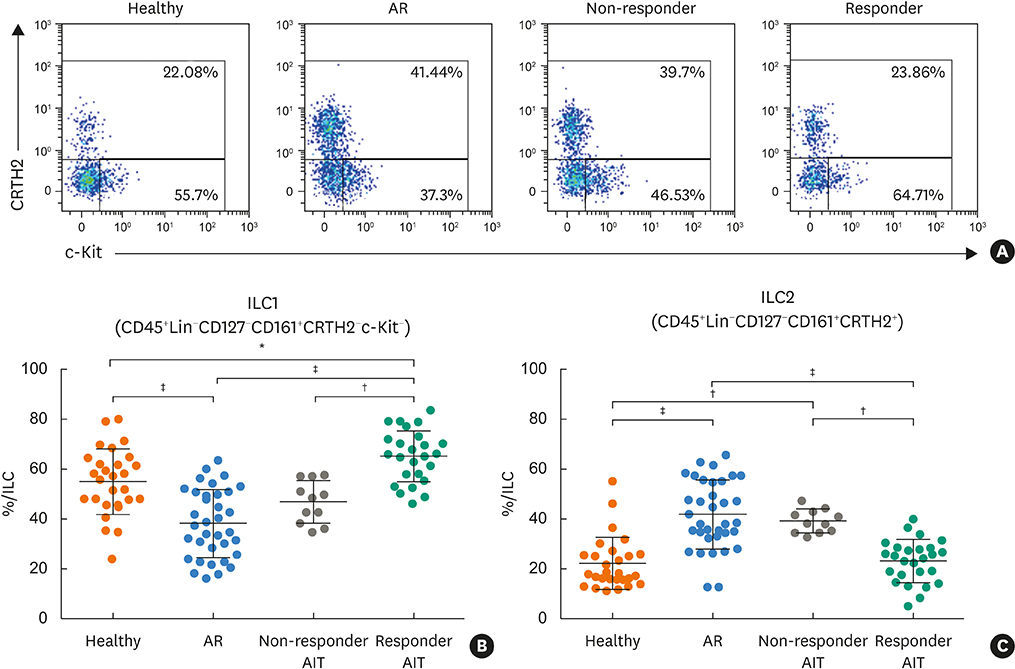
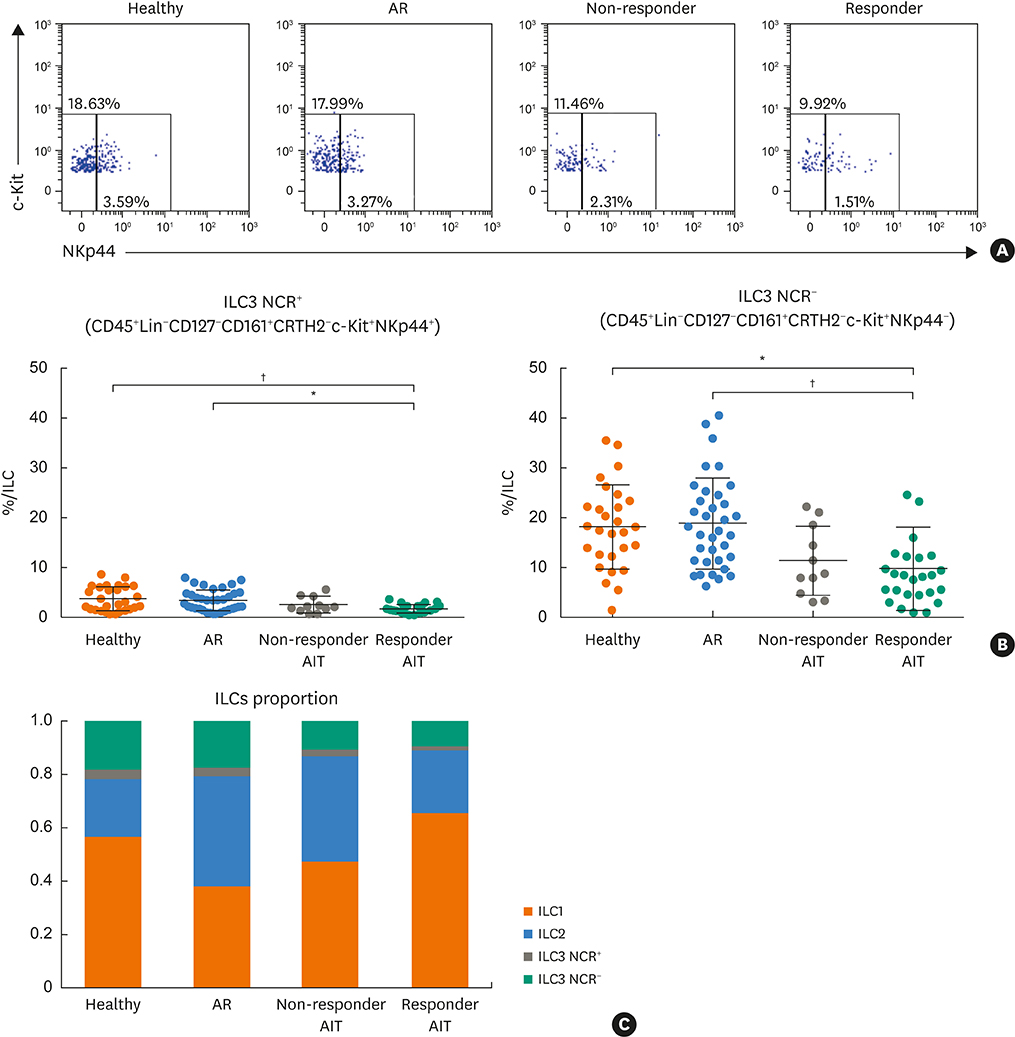
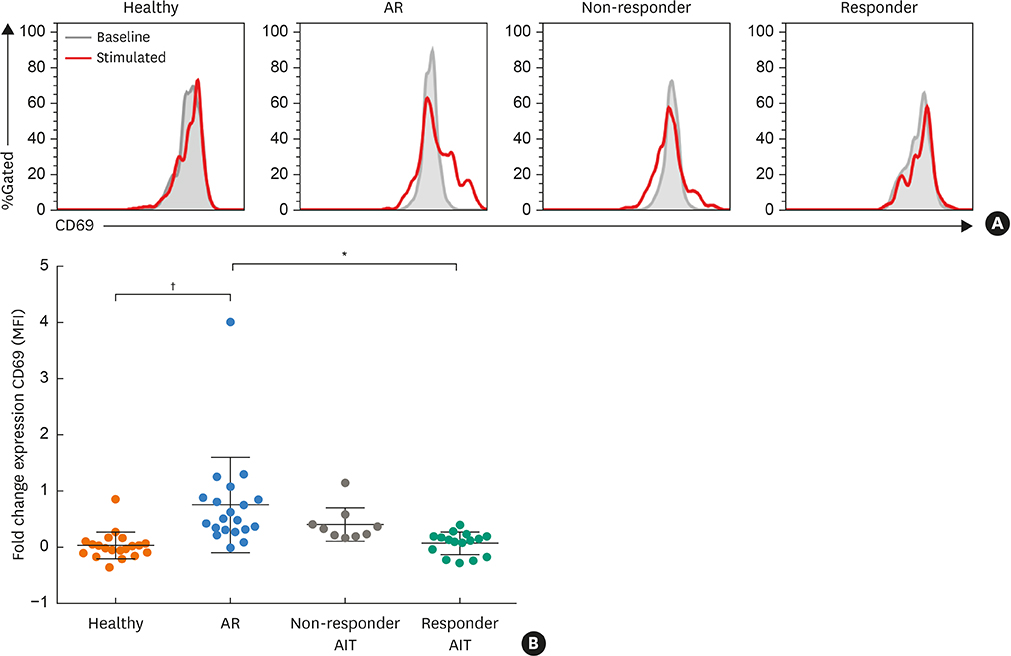
Responder AIT patients and healthy subjects had a decreased frequency of circulating ILC2s compared to non-responder AIT and AR patients. Conversely, ILC1s from responder AIT patients and healthy subjects showed increased frequency compared to non-responder AIT and AR patients. The frequency of ILC3s natural cytotoxicity receptor (NCR)+ and NCR− in responder AIT patients was significantly lower compared to AR patients and healthy subjects. The ILC1: ILC2 proportion in responder AIT patients was similar to that of healthy subjects. PBMCs from patients who were responders to AIT had a significantly lower expression of the activation marker CD69 on ILC2s in response to allergen re-stimulation compared to AR patients, but no difference compared to non-responder AIT patients and healthy subjects.
CONCLUSIONS
We propose that AIT might affect ILC responses. The activation of ILC2s was reduced in AR patients treated with AIT. Our results indicate that a relative ILC1/ILC2 skewed response is a possible key to successful AIT.
Keyword
MeSH Terms
Figure
Reference
-
1. Morita H, Moro K, Koyasu S. Innate lymphoid cells in allergic and nonallergic inflammation. J Allergy Clin Immunol. 2016; 138:1253–1264.
Article2. Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013; 13:145–149.3. Bernink JH, Peters CP, Munneke M, te Velde AA, Meijer SL, Weijer K, et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol. 2013; 14:221–229.
Article4. Fuchs A, Vermi W, Lee JS, Lonardi S, Gilfillan S, Newberry RD, et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-γ-producing cells. Immunity. 2013; 38:769–781.
Article5. Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature. 2010; 463:540–544.6. Mjösberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011; 12:1055–1062.
Article7. Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010; 107:11489–11494.
Article8. Mjösberg J, Bernink J, Golebski K, Karrich JJ, Peters CP, Blom B, et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012; 37:649–659.
Article9. Pelly VS, Kannan Y, Coomes SM, Entwistle LJ, Rückerl D, Seddon B, et al. IL-4-producing ILC2s are required for the differentiation of TH2 cells following Heligmosomoides polygyrus infection. Mucosal Immunol. 2016; 9:1407–1417.10. Melo-Gonzalez F, Hepworth MR. Functional and phenotypic heterogeneity of group 3 innate lymphoid cells. Immunology. 2017; 150:265–275.
Article11. Bartemes KR, Kephart GM, Fox SJ, Kita H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol. 2014; 134:671–678.e4.
Article12. Liu T, Wu J, Zhao J, Wang J, Zhang Y, Liu L, et al. Type 2 innate lymphoid cells: a novel biomarker of eosinophilic airway inflammation in patients with mild to moderate asthma. Respir Med. 2015; 109:1391–1396.
Article13. Smith SG, Chen R, Kjarsgaard M, Huang C, Oliveria JP, O'Byrne PM, et al. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol. 2016; 137:75–86.e8.
Article14. Nagakumar P, Denney L, Fleming L, Bush A, Lloyd CM, Saglani S. Type 2 innate lymphoid cells in induced sputum from children with severe asthma. J Allergy Clin Immunol. 2016; 137:624–626.e6.
Article15. Morita H, Arae K, Unno H, Miyauchi K, Toyama S, Nambu A, et al. An interleukin-33-mast cell-interleukin-2 axis suppresses papain-induced allergic inflammation by promoting regulatory T cell numbers. Immunity. 2015; 43:175–186.
Article16. Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012; 36:451–463.
Article17. Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage−CD25+CD44hi lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012; 188:1503–1513.18. Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011; 12:631–638.
Article19. Kim DW, Cho SH. Emerging endotypes of chronic rhinosinusitis and its application to precision medicine. Allergy Asthma Immunol Res. 2017; 9:299–306.
Article20. Doherty TA, Scott D, Walford HH, Khorram N, Lund S, Baum R, et al. Allergen challenge in allergic rhinitis rapidly induces increased peripheral blood type 2 innate lymphoid cells that express CD84. J Allergy Clin Immunol. 2014; 133:1203–1205.e7.
Article21. Lao-Araya M, Steveling E, Scadding GW, Durham SR, Shamji MH. Seasonal increases in peripheral innate lymphoid type 2 cells are inhibited by subcutaneous grass pollen immunotherapy. J Allergy Clin Immunol. 2014; 134:1193–1195.e4.
Article22. Lombardi V, Beuraud C, Neukirch C, Moussu H, Morizur L, Horiot S, et al. Circulating innate lymphoid cells are differentially regulated in allergic and nonallergic subjects. J Allergy Clin Immunol. 2016; 138:305–308.
Article23. Zhong H, Fan XL, Yu QN, Qin ZL, Chen D, Xu R, et al. Increased innate type 2 immune response in house dust mite-allergic patients with allergic rhinitis. Clin Immunol. 2017; 183:293–299.
Article24. Fan D, Wang X, Wang M, Wang Y, Zhang L, Li Y, et al. Allergen-dependent differences in ILC2s frequencies in patients with allergic rhinitis. Allergy Asthma Immunol Res. 2016; 8:216–222.
Article25. Caraballo L, Zakzuk J, Lee BW, Acevedo N, Soh JY, Sánchez-Borges M, et al. Particularities of allergy in the Tropics. World Allergy Organ J. 2016; 9:20.
Article26. Tham EH, Lee AJ, Bever HV. Aeroallergen sensitization and allergic disease phenotypes in Asia. Asian Pac J Allergy Immunol. 2016; 34:181–189.
Article27. Wise SK, Lin SY, Toskala E, Orlandi RR, Akdis CA, Alt JA, et al. International consensus statement on allergy and rhinology: allergic rhinitis. Int Forum Allergy Rhinol. 2018; 8:108–352.28. Calderón MA, Bousquet J, Canonica GW, Cardell LO, Fernandez de Rojas DH, Kleine-Tebbe J, et al. Guideline recommendations on the use of allergen immunotherapy in house dust mite allergy: Time for a change? J Allergy Clin Immunol. 2017; 140:41–52.
Article29. Jutel M, Kosowska A, Smolinska S. Allergen immunotherapy: past, present, and future. Allergy Asthma Immunol Res. 2016; 8:191–197.
Article30. Fan DC, Wang XD, Wang CS, Wang Y, Cao FF, Zhang L. Suppression of Immunotherapy on group 2 innate lymphoid cells in allergic rhinitis. Chin Med J (Engl). 2016; 129:2824–2828.
Article31. Cosmi L, Liotta F, Maggi L, Annunziato F. Role of type 2 innate lymphoid cells in allergic diseases. Curr Allergy Asthma Rep. 2017; 17:66.
Article32. Xu G, Zhang L, Wang DY, Xu R, Liu Z, Han DM, et al. Opposing roles of IL-17A and IL-25 in the regulation of TSLP production in human nasal epithelial cells. Allergy. 2010; 65:581–589.
Article33. Asaka D, Yoshikawa M, Nakayama T, Yoshimura T, Moriyama H, Otori N. Elevated levels of interleukin-33 in the nasal secretions of patients with allergic rhinitis. Int Arch Allergy Immunol. 2012; 158:Suppl 1. 47–50.
Article34. Kato Y, Akasaki S, Muto-Haenuki Y, Fujieda S, Matsushita K, Yoshimoto T. Nasal sensitization with ragweed pollen induces local-allergic-rhinitis-like symptoms in mice. PLoS One. 2014; 9:e103540.
Article35. Sohn MH. Efficacy and safety of subcutaneous allergen immunotherapy for allergic rhinitis. Allergy Asthma Immunol Res. 2018; 10:1–3.
Article36. Park KH, Son M, Choi SY, Park HJ, Lee JH, Jeong KY, et al. Erratum: In vitro evaluation of allergen potencies of commercial house dust mite sublingual immunotherapy reagents. Allergy Asthma Immunol Res. 2017; 9:187.37. Lee JH, Kim SC, Choi H, Jung CG, Ban GY, Shin YS, et al. A retrospective study of clinical response predictors in subcutaneous allergen immunotherapy with house dust mites for allergic rhinitis. Allergy Asthma Immunol Res. 2018; 10:18–24.
Article38. Smarr CB, Bryce PJ, Miller SD. Antigen-specific tolerance in immunotherapy of Th2-associated allergic diseases. Crit Rev Immunol. 2013; 33:389–414.
Article39. Wawrzyniak M, O'Mahony L, Akdis M. Role of regulatory cells in oral tolerance. Allergy Asthma Immunol Res. 2017; 9:107–115.
Article40. Kuipers H, Heirman C, Hijdra D, Muskens F, Willart M, van Meirvenne S, et al. Dendritic cells retrovirally overexpressing IL-12 induce strong Th1 responses to inhaled antigen in the lung but fail to revert established Th2 sensitization. J Leukoc Biol. 2004; 76:1028–1038.
Article41. Krishnamoorthy N, Burkett PR, Dalli J, Abdulnour RE, Colas R, Ramon S, et al. Cutting edge: maresin-1 engages regulatory T cells to limit type 2 innate lymphoid cell activation and promote resolution of lung inflammation. J Immunol. 2015; 194:863–867.
Article42. Rigas D, Lewis G, Aron JL, Wang B, Banie H, Sankaranarayanan I, et al. Type 2 innate lymphoid cell suppression by regulatory T cells attenuates airway hyperreactivity and requires inducible T-cell costimulator-inducible T-cell costimulator ligand interaction. J Allergy Clin Immunol. 2017; 139:1468–1477.e2.43. Doherty TA, Broide DH. Group 2 innate lymphoid cells: new players in human allergic diseases. J Investig Allergol Clin Immunol. 2015; 25:1–11.44. Bal SM, Bernink JH, Nagasawa M, Groot J, Shikhagaie MM, Golebski K, et al. IL-1β, IL-4 and IL-12 control the fate of group 2 innate lymphoid cells in human airway inflammation in the lungs. Nat Immunol. 2016; 17:636–645.
Article45. Li CW, Lu HG, Chen DH, Lin ZB, Wang DY, Li TY. In vivo and in vitro studies of Th17 response to specific immunotherapy in house dust mite-induced allergic rhinitis patients. PLoS One. 2014; 9:e91950.46. Gueguen C, Bouley J, Moussu H, Luce S, Duchateau M, Chamot-Rooke J, et al. Changes in markers associated with dendritic cells driving the differentiation of either TH2 cells or regulatory T cells correlate with clinical benefit during allergen immunotherapy. J Allergy Clin Immunol. 2016; 137:545–558.47. von Burg N, Turchinovich G, Finke D. Maintenance of immune homeostasis through ILC/T cell interactions. Front Immunol. 2015; 6:416.
Article48. Sugita K, Steer CA, Martinez-Gonzalez I, Altunbulakli C, Morita H, Castro-Giner F, et al. Type 2 innate lymphoid cells disrupt bronchial epithelial barrier integrity by targeting tight junctions through IL-13 in asthmatic patients. J Allergy Clin Immunol. 2018; 141:300–310.e11.
Article49. Kelly E, Won A, Refaeli Y, Van Parijs L. IL-2 and related cytokines can promote T cell survival by activating AKT. J Immunol. 2002; 168:597–603.
Article50. Seehus CR, Kadavallore A, Torre B, Yeckes AR, Wang Y, Tang J, et al. Alternative activation generates IL-10 producing type 2 innate lymphoid cells. Nat Commun. 2017; 8:1900.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Update in the Mechanisms of Allergen-Specific Immunotheraphy
- Allergen Specific Immunotherapy in Allergic Rhinitis
- Allergen specific IgG antibodies in nasal secretion during allergen specific immunotherapy
- Sublingual immunotherapy for allergic rhinitis
- The effect of house dust mite conventional immunotherapy on the production of IL-4 and interferon-gamma from the peripheral blood T cells in asthmatic children