Nutr Res Pract.
2016 Aug;10(4):386-392. 10.4162/nrp.2016.10.4.386.
Effects of disturbed liver growth and oxidative stress of high-fat diet-fed dams on cholesterol metabolism in offspring mice
- Affiliations
-
- 1Department of Food and Nutrition, Seoul National University, 1 Gwanak-ro, Gwanak-gu, Seoul 08826, Korea. hye0414@snu.ac.kr
- 2Research Institute of Human Ecology, Seoul National University, Seoul 08826, Korea.
- KMID: 2390140
- DOI: http://doi.org/10.4162/nrp.2016.10.4.386
Abstract
- BACKGROUND/OBJECTIVES
Changes in nutritional status during gestation and lactation have detrimental effects on offspring metabolism. Several animal studies have shown that maternal high-fat diet (HFD) can predispose the offspring to development of obesity and metabolic diseases, however the mechanisms underlying these transgenerational effects are poorly understood. Therefore, we examined the effect of maternal HFD consumption on metabolic phenotype and hepatic expression of involved genes in dams to determine whether any of these parameters were associated with the metabolic outcomes in the offspring.
MATERIALS/METHODS
Female C57BL/6 mice were fed a low-fat diet (LFD: 10% calories from fat) or a high-fat diet (HFD: 45% calories from fat) for three weeks before mating, and during pregnancy and lactation. Dams and their male offspring were studied at weaning.
RESULTS
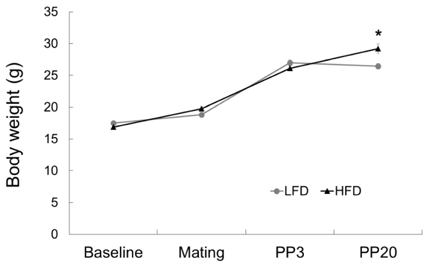
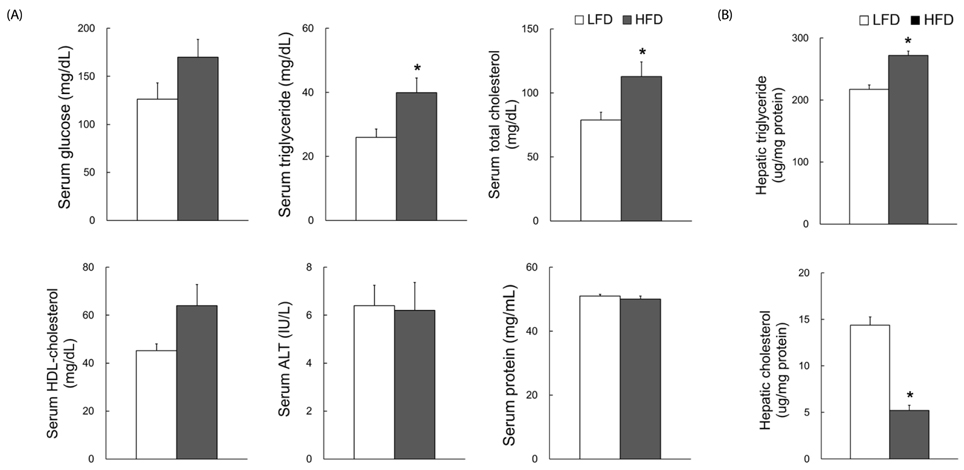
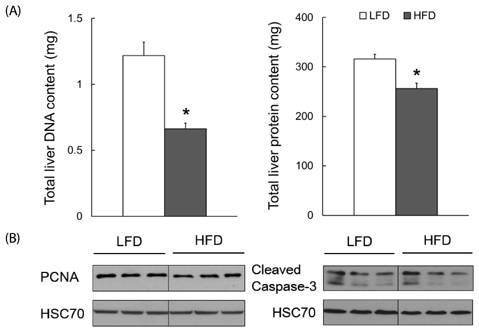
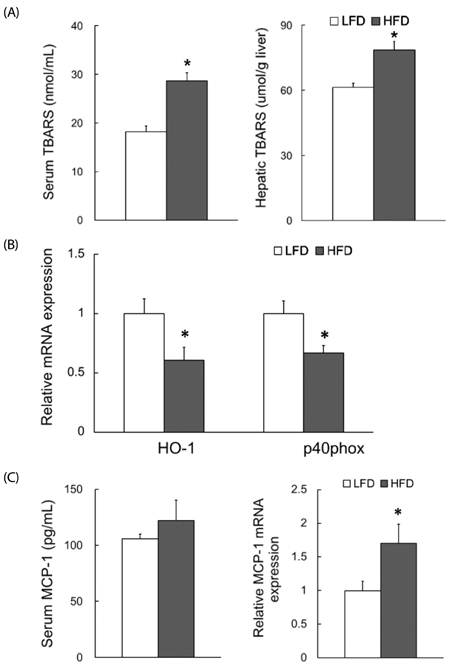
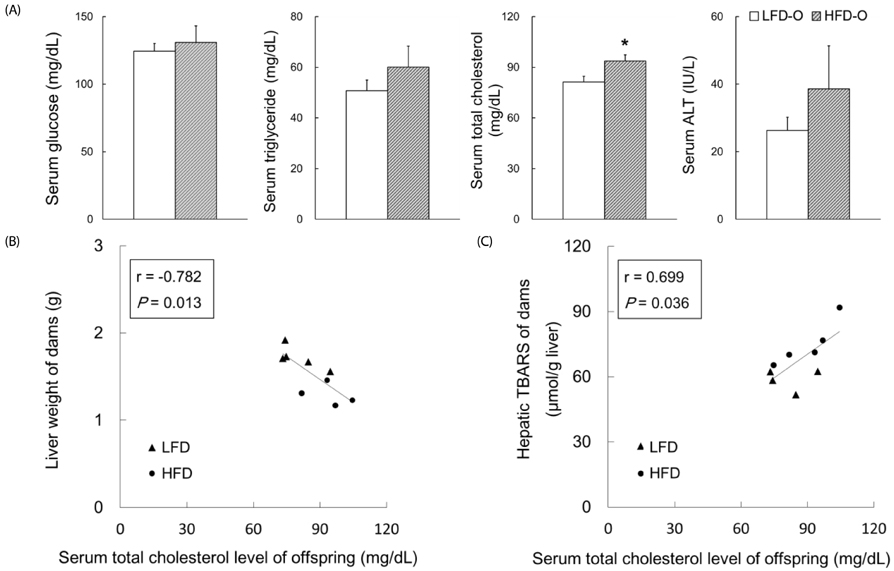
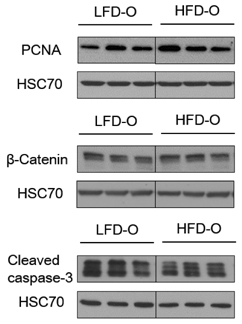
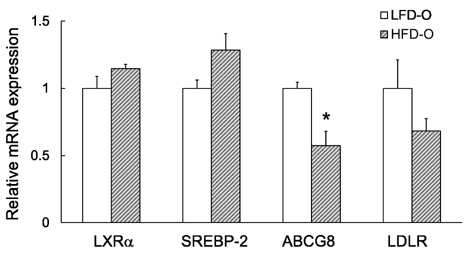
Dams fed an HFD had significantly higher body and adipose tissue weights and higher serum triglyceride and cholesterol levels than dams fed an LFD. Hepatic lipid levels and mRNA levels of genes involved in lipid metabolism, including LXRα, SREBP-2, FXR, LDLR, and ABCG8 were significantly changed by maternal HFD intake. Significantly lower total liver DNA and protein contents were observed in dams fed an HFD, implicating the disturbed liver adaptation in the pregnancy-related metabolic demand. HFD feeding also induced significant oxidative stress in serum and liver of dams. Offspring of dams fed an HFD had significantly higher serum cholesterol levels, which were negatively correlated with liver weights of dams and positively correlated with hepatic lipid peroxide levels in dams.
CONCLUSIONS
Maternal HFD consumption induced metabolic dysfunction, including altered liver growth and oxidative stress in dams, which may contribute to the disturbed cholesterol homeostasis in the early life of male mice offspring.
MeSH Terms
-
Adipose Tissue
Animals
Cholesterol*
Diet, Fat-Restricted
Diet, High-Fat
DNA
Female
Homeostasis
Humans
Lactation
Lipid Metabolism
Liver*
Male
Metabolic Diseases
Metabolism*
Mice*
Nutritional Status
Obesity
Oxidative Stress*
Phenotype
Pregnancy
RNA, Messenger
Triglycerides
Weaning
Weights and Measures
Cholesterol
DNA
RNA, Messenger
Figure
Cited by 1 articles
-
Erratum: Effects of disturbed liver growth and oxidative stress of high-fat diet-fed dams on cholesterol metabolism in offspring mice
Juyoung Kim, Juhae Kim, Young Hye Kwon
Nutr Res Pract. 2017;11(5):435-435. doi: 10.4162/nrp.2017.11.5.435.
Reference
-
1. Burdge GC, Lillycrop KA. Nutrition, epigenetics, and developmental plasticity: implications for understanding human disease. Annu Rev Nutr. 2010; 30:315–339.
Article2. Rinaudo P, Wang E. Fetal programming and metabolic syndrome. Annu Rev Physiol. 2012; 74:107–130.
Article3. Williams L, Seki Y, Vuguin PM, Charron MJ. Animal models of in utero exposure to a high fat diet: a review. Biochim Biophys Acta. 2014; 1842:507–519.
Article4. Cannon MV, Buchner DA, Hester J, Miller H, Sehayek E, Nadeau JH, Serre D. Maternal nutrition induces pervasive gene expression changes but no detectable DNA methylation differences in the liver of adult offspring. PLoS One. 2014; 9:e90335.
Article5. Cianfarani S, Agostoni C, Bedogni G, Berni Canani R, Brambilla P, Nobili V, Pietrobelli A. Effect of intrauterine growth retardation on liver and long-term metabolic risk. Int J Obes (Lond). 2012; 36:1270–1277.
Article6. Heerwagen MJ, Miller MR, Barbour LA, Friedman JE. Maternal obesity and fetal metabolic programming: a fertile epigenetic soil. Am J Physiol Regul Integr Comp Physiol. 2010; 299:R711–R722.
Article7. Bringhenti I, Ornellas F, Martins MA, Mandarim-de-Lacerda CA, Aguila MB. Early hepatic insult in the offspring of obese maternal mice. Nutr Res. 2015; 35:136–145.
Article8. Yang KF, Cai W, Xu JL, Shi W. Maternal high-fat diet programs Wnt genes through histone modification in the liver of neonatal rats. J Mol Endocrinol. 2012; 49:107–114.
Article9. Zheng J, Xiao X, Zhang Q, Yu M, Xu J, Wang Z. Maternal high-fat diet modulates hepatic glucose, lipid homeostasis and gene expression in the PPAR pathway in the early life of offspring. Int J Mol Sci. 2014; 15:14967–14983.
Article10. Zou Y, Hu M, Bao Q, Chan JY, Dai G. Nrf2 participates in regulating maternal hepatic adaptations to pregnancy. J Cell Sci. 2013; 126:1618–1625.
Article11. Herrera E. Metabolic adaptations in pregnancy and their implications for the availability of substrates to the fetus. Eur J Clin Nutr. 2000; 54:Suppl 1. S47–S51.
Article12. Bustamante JJ, Copple BL, Soares MJ, Dai G. Gene profiling of maternal hepatic adaptations to pregnancy. Liver Int. 2010; 30:406–415.
Article13. Papacleovoulou G, Abu-Hayyeh S, Williamson C. Nuclear receptor-driven alterations in bile acid and lipid metabolic pathways during gestation. Biochim Biophys Acta. 2011; 1812:879–887.
Article14. Athippozhy A, Huang L, Wooton-Kee CR, Zhao T, Jungsuwadee P, Stromberg AJ, Vore M. Differential gene expression in liver and small intestine from lactating rats compared to age-matched virgin controls detects increased mRNA of cholesterol biosynthetic genes. BMC Genomics. 2011; 12:95.
Article15. Niculescu MD, Lupu DS. High fat diet-induced maternal obesity alters fetal hippocampal development. Int J Dev Neurosci. 2009; 27:627–633.
Article16. Shaw MA, Rasmussen KM, Myers TR. Consumption of a high fat diet impairs reproductive performance in Sprague-Dawley rats. J Nutr. 1997; 127:64–69.
Article17. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95:351–358.
Article18. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957; 226:497–509.
Article19. Jeon S, Park YJ, Kwon YH. Genistein alleviates the development of nonalcoholic steatohepatitis in ApoE(-/-) mice fed a high-fat diet. Mol Nutr Food Res. 2014; 58:830–841.
Article20. Flowers JB, Rabaglia ME, Schueler KL, Flowers MT, Lan H, Keller MP, Ntambi JM, Attie AD. Loss of stearoyl-CoA desaturase-1 improves insulin sensitivity in lean mice but worsens diabetes in leptin-deficient obese mice. Diabetes. 2007; 56:1228–1239.
Article21. Matsui H, Yokoyama T, Sekiguchi K, Iijima D, Sunaga H, Maniwa M, Ueno M, Iso T, Arai M, Kurabayashi M. Stearoyl-CoA desaturase-1 (SCD1) augments saturated fatty acid-induced lipid accumulation and inhibits apoptosis in cardiac myocytes. PLoS One. 2012; 7:e33283.
Article22. Dudley KJ, Sloboda DM, Connor KL, Beltrand J, Vickers MH. Offspring of mothers fed a high fat diet display hepatic cell cycle inhibition and associated changes in gene expression and DNA methylation. PLoS One. 2011; 6:e21662.
Article23. Mischke M, Pruis MG, Boekschoten MV, Groen AK, Fitri AR, van de Heijning BJ, Verkade HJ, Müller M, Plösch T, Steegenga WT. Maternal Western-style high fat diet induces sex-specific physiological and molecular changes in two-week-old mouse offspring. PLoS One. 2013; 8:e78623.
Article24. Al-Gubory KH, Fowler PA, Garrel C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol. 2010; 42:1634–1650.
Article25. Sen S, Simmons RA. Maternal antioxidant supplementation prevents adiposity in the offspring of Western diet-fed rats. Diabetes. 2010; 59:3058–3065.
Article26. Baardman ME, Kerstjens-Frederikse WS, Berger RM, Bakker MK, Hofstra RM, Plösch T. The role of maternal-fetal cholesterol transport in early fetal life: current insights. Biol Reprod. 2013; 88:24.27. Ashino NG, Saito KN, Souza FD, Nakutz FS, Roman EA, Velloso LA, Torsoni AS, Torsoni MA. Maternal high-fat feeding through pregnancy and lactation predisposes mouse offspring to molecular insulin resistance and fatty liver. J Nutr Biochem. 2012; 23:341–348.
Article28. Hernandez LL, Grayson BE, Yadav E, Seeley RJ, Horseman ND. High fat diet alters lactation outcomes: possible involvement of inflammatory and serotonergic pathways. PLoS One. 2012; 7:e32598.
Article29. Apte U, Zeng G, Thompson MD, Muller P, Micsenyi A, Cieply B, Kaestner KH, Monga SP. beta-Catenin is critical for early postnatal liver growth. Am J Physiol Gastrointest Liver Physiol. 2007; 292:G1578–G1585.30. Ito J, Nakagawa K, Kato S, Miyazawa T, Kimura F, Miyazawa T. The combination of maternal and offspring high-fat diets causes marked oxidative stress and development of metabolic syndrome in mouse offspring. Life Sci. 2016; 151:70–75.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Effects of disturbed liver growth and oxidative stress of high-fat diet-fed dams on cholesterol metabolism in offspring mice
- Effects of d-alpha-tocopherol supplements on lipid metabolism in a high-fat diet-fed animal model
- Effect of combined mulberry leaf and fruit extract on liver and skin cholesterol transporters in high fat diet-induced obese mice
- Effects of Liquid Culture of Coriolus Versicolor on Lipid Metabolism and Enzyme Activities in Rats Fed High Fat Diet
- Anti-obesity Effect of Steamed Soybean and Fermented Steamed Soybean in High-fat Diet-induced Obese ICR Mice