Endocrinol Metab.
2017 Sep;32(3):375-382. 10.3803/EnM.2017.32.3.375.
A Novel Index Using Soluble CD36 Is Associated with the Prevalence of Type 2 Diabetes Mellitus: Comparison Study with Triglyceride-Glucose Index
- Affiliations
-
- 1Department of Internal Medicine, Yeungnam University College of Medicine, Daegu, Korea. helee@ynu.ac.kr
- KMID: 2389819
- DOI: http://doi.org/10.3803/EnM.2017.32.3.375
Abstract
- BACKGROUND
Plasma soluble cluster determinant 36 (sCD36) level is closely related with insulin resistance and atherosclerosis, but little is known whether it could be a surrogate for estimating risk of developing diabetes or not. To address this, we evaluated association between sCD36 index, the product of sCD36 and fasting plasma glucose (FPG), and the prevalence of type 2 diabetes mellitus (T2DM), and then compared with triglyceride-glucose (TyG) index which has been suggested simple index for insulin resistance.
METHODS
This was cross-sectional study, and participants were classified as normal glucose tolerance (NGT), prediabetes, and T2DM according to glucose tolerance. The formula of TyG index was "˜ln [FPG (mg/dL)×triglyceride (mg/dL)/2],' and the sCD36 index was "˜ln [sCD36 (pg/mL)×FPG (mg/dL)/2].'
RESULTS
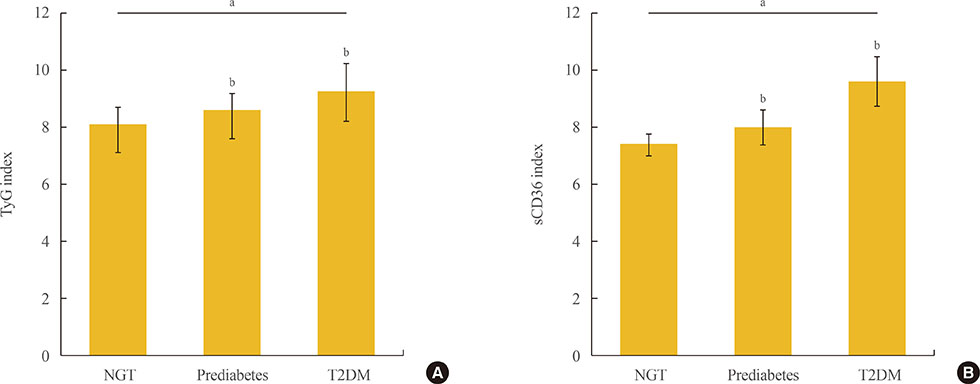
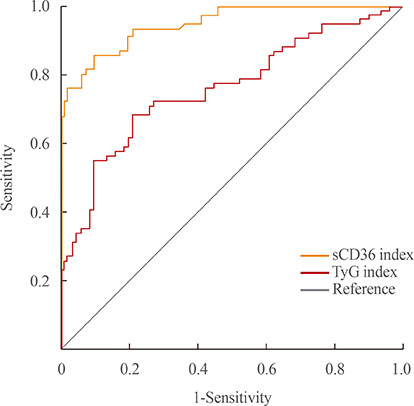
One hundred and fifty-five subjects (mean age, 55.2 years) were enrolled, and patients with T2DM were 75. Both indexes were significantly increased in prediabetes and T2DM rather than NGT, and sCD36 index was positively correlated with both glycosylated hemoglobin and homeostasis model assessment of insulin resistance (r=0.767 and r=0.453, respectively; P<0.05) and negatively with homeostasis model assessment estimate of β-cell function (r=−0.317). The odds ratio (OR) of sCD36 index for T2DM was 4.39 (95% confidential interval, 1.51 to 12.77) after adjusting age, gender, blood pressure, smoking, alcohol, non-high density lipoprotein cholesterol and high-sensitivity C-reactive protein. However, OR of TyG index did not remained significance after adjustment.
CONCLUSION
sCD36 index has an independent association with the risk of T2DM, and showed better correlation than TyG index. These results suggest sCD36 index might be useful surrogate marker for the risk of diabetes.
MeSH Terms
-
Atherosclerosis
Biomarkers
Blood Glucose
Blood Pressure
C-Reactive Protein
Cholesterol
Cross-Sectional Studies
Diabetes Mellitus, Type 2*
Fasting
Glucose
Hemoglobin A, Glycosylated
Homeostasis
Humans
Insulin Resistance
Lipoproteins
Odds Ratio
Plasma
Prediabetic State
Prevalence*
Smoke
Smoking
Biomarkers
C-Reactive Protein
Cholesterol
Glucose
Lipoproteins
Smoke
Figure
Cited by 2 articles
-
The Role of CD36 in Type 2 Diabetes Mellitus: β-Cell Dysfunction and Beyond
Jun Sung Moon, Udayakumar Karunakaran, Elumalai Suma, Seung Min Chung, Kyu Chang Won
Diabetes Metab J. 2020;44(2):222-233. doi: 10.4093/dmj.2020.0053.Overcoming β-Cell Dysfunction in Type 2 Diabetes Mellitus: CD36 Inhibition and Antioxidant System
Il Rae Park, Yong Geun Chung, Kyu Chang Won
Diabetes Metab J. 2025;49(1):1-12. doi: 10.4093/dmj.2024.0796.
Reference
-
1. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010; 87:4–14.2. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004; 27:1047–1053.3. Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care. 2000; 23:465–471.4. Ford ES. Body mass index, diabetes, and C-reactive protein among U.S. adults. Diabetes Care. 1999; 22:1971–1977.5. Guerrero-Romero F, Simental-Mendia LE, Gonzalez-Ortiz M, Martinez-Abundis E, Ramos-Zavala MG, Hernandez-Gonzalez SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010; 95:3347–3351.6. Du T, Yuan G, Zhang M, Zhou X, Sun X, Yu X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol. 2014; 13:146.7. Abbasi F, Reaven GM. Comparison of two methods using plasma triglyceride concentration as a surrogate estimate of insulin action in nondiabetic subjects: triglycerides × glucose versus triglyceride/high-density lipoprotein cholesterol. Metabolism. 2011; 60:1673–1676.8. Lee SH, Kwon HS, Park YM, Ha HS, Jeong SH, Yang HK, et al. Predicting the development of diabetes using the product of triglycerides and glucose: the Chungju Metabolic Disease Cohort (CMC) study. PLoS One. 2014; 9:e90430.9. Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008; 6:299–304.10. Vasques AC, Novaes FS, de Oliveira Mda S, Souza JR, Yamanaka A, Pareja JC, et al. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011; 93:e98–e100.11. Febbraio M, Hajjar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest. 2001; 108:785–791.12. Moon JS, Karunakaran U, Elumalai S, Lee IK, Lee HW, Kim YW, et al. Metformin prevents glucotoxicity by alleviating oxidative and ER stress-induced CD36 expression in pancreatic beta cells. J Diabetes Complications. 2017; 31:21–30.13. Kim YW, Moon JS, Seo YJ, Park SY, Kim JY, Yoon JS, et al. Inhibition of fatty acid translocase cluster determinant 36 (CD36), stimulated by hyperglycemia, prevents glucotoxicity in INS-1 cells. Biochem Biophys Res Commun. 2012; 420:462–466.14. Yoon JS, Moon JS, Kim YW, Won KC, Lee HW. The glucotoxicity protecting effect of ezetimibe in pancreatic beta cells via inhibition of CD36. J Korean Med Sci. 2016; 31:547–552.15. Karunakaran U, Moon JS, Lee HW, Won KC. CD36 initiated signaling mediates ceramide-induced TXNIP expression in pancreatic beta-cells. Biochim Biophys Acta. 2015; 1852:2414–2422.16. Collot-Teixeira S, Martin J, McDermott-Roe C, Poston R, McGregor JL. CD36 and macrophages in atherosclerosis. Cardiovasc Res. 2007; 75:468–477.17. Handberg A, Levin K, Hojlund K, Beck-Nielsen H. Identification of the oxidized low-density lipoprotein scavenger receptor CD36 in plasma: a novel marker of insulin resistance. Circulation. 2006; 114:1169–1176.18. Glintborg D, Hojlund K, Andersen M, Henriksen JE, Beck-Nielsen H, Handberg A. Soluble CD36 and risk markers of insulin resistance and atherosclerosis are elevated in polycystic ovary syndrome and significantly reduced during pioglitazone treatment. Diabetes Care. 2008; 31:328–334.19. Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015; 38:140–149.20. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28:412–419.21. Handberg A, Hojlund K, Gastaldelli A, Flyvbjerg A, Dekker JM, Petrie J, et al. Plasma sCD36 is associated with markers of atherosclerosis, insulin resistance and fatty liver in a nondiabetic healthy population. J Intern Med. 2012; 271:294–304.22. Handberg A, Norberg M, Stenlund H, Hallmans G, Attermann J, Eriksson JW. Soluble CD36 (sCD36) clusters with markers of insulin resistance, and high sCD36 is associated with increased type 2 diabetes risk. J Clin Endocrinol Metab. 2010; 95:1939–1946.23. Chmielewski M, Bragfors-Helin AC, Stenvinkel P, Lindholm B, Anderstam B. Serum soluble CD36, assessed by a novel monoclonal antibody-based sandwich ELISA, predicts cardiovascular mortality in dialysis patients. Clin Chim Acta. 2010; 411:2079–2082.24. Han CY. Roles of reactive oxygen species on insulin resistance in adipose tissue. Diabetes Metab J. 2016; 40:272–279.25. Bonen A, Tandon NN, Glatz JF, Luiken JJ, Heigenhauser GJ. The fatty acid transporter FAT/CD36 is upregulated in subcutaneous and visceral adipose tissues in human obesity and type 2 diabetes. Int J Obes (Lond). 2006; 30:877–883.26. Aguer C, Mercier J, Man CY, Metz L, Bordenave S, Lambert K, et al. Intramyocellular lipid accumulation is associated with permanent relocation ex vivo and in vitro of fatty acid translocase (FAT)/CD36 in obese patients. Diabetologia. 2010; 53:1151–1163.27. Paolisso G, Tataranni PA, Foley JE, Bogardus C, Howard BV, Ravussin E. A high concentration of fasting plasma non-esterified fatty acids is a risk factor for the development of NIDDM. Diabetologia. 1995; 38:1213–1217.28. Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes. 1995; 44:863–870.29. Zhang HM, Zhang XL, Zhou X, Li D, Gu JG, Wu JJ. Mechanism linking atherosclerosis and type 2 diabetes: increased expression of scavenger receptor CD36 in monocytes. Chin Med J (Engl). 2005; 118:1717–1722.30. Kennedy DJ, Kashyap SR. Pathogenic role of scavenger receptor CD36 in the metabolic syndrome and diabetes. Metab Syndr Relat Disord. 2011; 9:239–245.31. Zhou J, Febbraio M, Wada T, Zhai Y, Kuruba R, He J, et al. Hepatic fatty acid transporter CD36 is a common target of LXR, PXR, and PPARgamma in promoting steatosis. Gastroenterology. 2008; 134:556–567.32. Koonen DP, Jacobs RL, Febbraio M, Young ME, Soltys CL, Ong H, et al. Increased hepatic CD36 expression contributes to dyslipidemia associated with diet-induced obesity. Diabetes. 2007; 56:2863–2871.33. Bonen A, Luiken JJ, Arumugam Y, Glatz JF, Tandon NN. Acute regulation of fatty acid uptake involves the cellular redistribution of fatty acid translocase. J Biol Chem. 2000; 275:14501–14508.34. Alkhatatbeh MJ, Enjeti AK, Acharya S, Thorne RF, Lincz LF. The origin of circulating CD36 in type 2 diabetes. Nutr Diabetes. 2013; 3:e59.35. Shiju TM, Mohan V, Balasubramanyam M, Viswanathan P. Soluble CD36 in plasma and urine: a plausible prognostic marker for diabetic nephropathy. J Diabetes Complications. 2015; 29:400–406.36. Handberg A, Lopez-Bermejo A, Bassols J, Vendrell J, Ricart W, Fernandez-Real JM. Circulating soluble CD36 is associated with glucose metabolism and interleukin-6 in glucose-intolerant men. Diab Vasc Dis Res. 2009; 6:15–20.37. Navarro-Gonzalez D, Sanchez-Inigo L, Pastrana-Delgado J, Fernandez-Montero A, Martinez JA. Triglyceride-glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: the Vascular-Metabolic CUN cohort. Prev Med. 2016; 86:99–105.38. Janghorbani M, Almasi SZ, Amini M. The product of triglycerides and glucose in comparison with fasting plasma glucose did not improve diabetes prediction. Acta Diabetol. 2015; 52:781–788.39. Lee SH, Yang HK, Ha HS, Lee JH, Kwon HS, Park YM, et al. Changes in metabolic health status over time and risk of developing type 2 diabetes: a prospective cohort study. Medicine (Baltimore). 2015; 94:e1705.40. Ginsberg HN, Zhang YL, Hernandez-Ono A. Regulation of plasma triglycerides in insulin resistance and diabetes. Arch Med Res. 2005; 36:232–240.41. Szapary PO, Bloedon LT, Foster GD. Physical activity and its effects on lipids. Curr Cardiol Rep. 2003; 5:488–492.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prevalence and Risk Factors for Diabetes Mellitus and Impaired Fasting Glucose of Adults
- Potential Association of Triglyceride Glucose Index with Cardiac Autonomic Neuropathy in Type 2 Diabetes Mellitus Patients
- The Role of CD36 in Type 2 Diabetes Mellitus: β-Cell Dysfunction and Beyond
- Comparison of the Efficacy of Dipeptidylpeptidase-4 Inhibitors Between Asian and Non-Asian Populations
- Prevalence and Clinical Characteristics of Aspirin Resistance in the Patients with Type 2 Diabetes Mellitus