J Korean Med Sci.
2017 Jul;32(7):1131-1138. 10.3346/jkms.2017.32.7.1131.
Potential Association of Triglyceride Glucose Index with Cardiac Autonomic Neuropathy in Type 2 Diabetes Mellitus Patients
- Affiliations
-
- 1Department of Pharmaceutical Medicine, Faculty of Pharmacy, Hamdard University, New Delhi, India.
- 2Department of Pharmacology, Faculty of Pharmacy, Hamdard University, New Delhi, India. uma_bora@hotmail.com
- 3Department of Medicine, Hamdard Institute of Medical Sciences and Research, Hamdard University, New Delhi, India.
- 4Department of Pharmacology, Hamdard Institute of Medical Sciences and Research, Hamdard University, New Delhi, India.
- KMID: 2379608
- DOI: http://doi.org/10.3346/jkms.2017.32.7.1131
Abstract
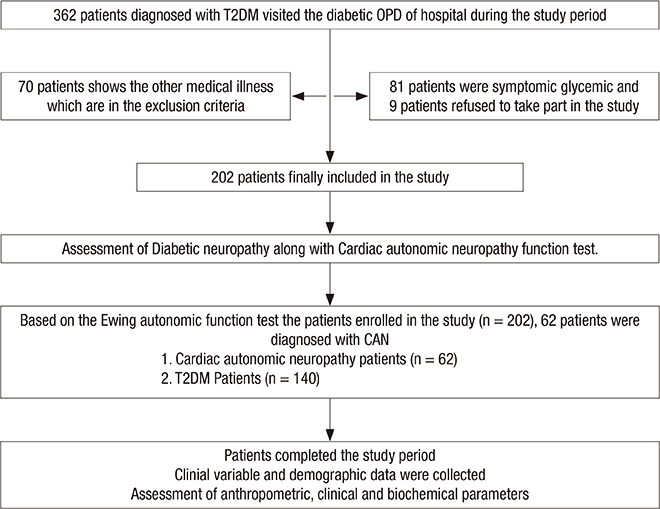
- Cardiac autonomic neuropathy (CAN) is a common and most neglected complication of diabetes, estimated to be roughly 8% in recently diagnosed patients and greater than 50% in patients with chronic disease history. The insulin resistance (IR) itself is bidirectionally associated with increased risk of type 2 diabetes mellitus (T2DM) and CAN is a predisposing factor. The primary objective of the present study was aimed to find a correlation of triglyceride glucose index (TyG index) in CAN patients along with the prevalence of CAN in T2DM patients as a secondary objective. This prevalence study was conducted on 202 patients visiting the diabetic clinic of Hamdard Institute of Medical Sciences and Research, Jamia Hamdard (HIMSR) teaching hospital in New Delhi, India who fulfilled the inclusion criteria. The Ewings autonomic function test was used for diagnosis of CAN. TyG index was calculated for patients based on fasting levels of glucose and triglyceride. The CAN was diagnosed in 62 participants out of 202 T2DM patients (overall prevalence 30.7%). The mean ± standard deviation (SD) for TyG index was 10.3 ± 0.2 and 9.5 ± 0.2 in CAN positive, T2DM patients, respectively. The difference of TyG index, in CAN positive and T2DM patients, was highly significant (P < 0.001). Further correlation analysis was performed to find an association of TyG index, duration, and age with patient groups. TyG index showed a positive correlation with heart rate during deep breathing (HRD), heart rate variation during standing (HRS), blood pressure (BP) response to handgrip and BP response to standing. Our finding highlights the TyG index, low-cost IR index, might be useful as an alternative tool for the early screening of patients at a high risk of diabetic neuropathy.
Keyword
MeSH Terms
Figure
Reference
-
1. Hinder LM, Vincent AM, Burant CF, Pennathur S, Feldman EL. Bioenergetics in diabetic neuropathy: what we need to know. J Peripher Nerv Syst. 2012; 17:Suppl 2. 10–14.2. Yagihashi S, Mizukami H, Sugimoto K. Mechanism of diabetic neuropathy: where are we now and where to go? J Diabetes Investig. 2011; 2:18–32.3. Kilpatrick ES, Rigby AS, Atkin SL. Insulin resistance, the metabolic syndrome, and complication risk in type 1 diabetes: “double diabetes” in the Diabetes Control and Complications Trial. Diabetes Care. 2007; 30:707–712.4. Choi SH, Hur KY, Kim DJ, Ahn CW, Kang ES, Cha BS, Lim SK, Huh KB, Lee HC. Staged diabetes management according to individual patient insulin resistance and beta-cell function ameliorates glycaemic control in type 2 diabetes mellitus. Clin Endocrinol (Oxf). 2008; 69:549–555.5. The effect of intensive diabetes therapy on measures of autonomic nervous system function in the Diabetes Control and Complications Trial (DCCT). Diabetologia. 1998; 41:416–423.6. Kuehl M, Stevens MJ. Cardiovascular autonomic neuropathies as complications of diabetes mellitus. Nat Rev Endocrinol. 2012; 8:405–416.7. Du T, Yuan G, Zhang M, Zhou X, Sun X, Yu X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol. 2014; 13:146.8. Miselli MA, Nora ED, Passaro A, Tomasi F, Zuliani G. Plasma triglycerides predict ten-years all-cause mortality in outpatients with type 2 diabetes mellitus: a longitudinal observational study. Cardiovasc Diabetol. 2014; 13:135.9. Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009; 302:1993–2000.10. Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011; 123:2292–2333.11. Turner RC. The U.K. prospective diabetes study. A review. Diabetes Care. 1998; 21:Suppl 3. C35–8.12. Lee SH, Han K, Yang HK, Kim HS, Cho JH, Kwon HS, Park YM, Cha BY, Yoon KH. A novel criterion for identifying metabolically obese but normal weight individuals using the product of triglycerides and glucose. Nutr Diabetes. 2015; 5:e149.13. Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008; 6:299–304.14. Lee SH, Kwon HS, Park YM, Ha HS, Jeong SH, Yang HK, Lee JH, Yim HW, Kang MI, Lee WC, et al. Predicting the development of diabetes using the product of triglycerides and glucose: the Chungju Metabolic Disease Cohort (CMC) study. PLoS One. 2014; 9:e90430.15. Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, Jacques-Camarena O, Rodríguez-Morán M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010; 95:3347–3351.16. Tomlin AM, Dovey SM, Tilyard MW. Risk factors for hospitalization due to diabetes complications. Diabetes Res Clin Pract. 2008; 80:244–252.17. Ewing DJ, Martyn CN, Young RJ, Clarke BF. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care. 1985; 8:491–498.18. Bellavere F, Bosello G, Fedele D, Cardone C, Ferri M. Diagnosis and management of diabetic autonomic neuropathy. Br Med J (Clin Res Ed). 1983; 287:61.19. Pappachan JM, Sebastian J, Bino BC, Jayaprakash K, Vijayakumar K, Sujathan P, Adinegara LA. Cardiac autonomic neuropathy in diabetes mellitus: prevalence, risk factors and utility of corrected QT interval in the ECG for its diagnosis. Postgrad Med J. 2008; 84:205–210.20. Turner N, Heilbronn LK. Is mitochondrial dysfunction a cause of insulin resistance? Trends Endocrinol Metab. 2008; 19:324–330.21. Takayama S, Sakura H, Katsumori K, Wasada T, Iwamoto Y. A possible involvement of parasympathetic neuropathy on insulin resistance in patients with type 2 diabetes. Diabetes Care. 2001; 24:968–969.22. Kempler P, Tesfaye S, Chaturvedi N, Stevens LK, Webb DJ, Eaton S, Kerényi Z, Tamás G, Ward JD, Fuller JH; EURODIAB IDDM Complications Study Group. Autonomic neuropathy is associated with increased cardiovascular risk factors: the EURODIAB IDDM Complications Study. Diabet Med. 2002; 19:900–909.23. Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 1993; 36:150–154.24. Dyck PJ, Davies JL, Litchy WJ, O’Brien PC. Longitudinal assessment of diabetic polyneuropathy using a composite score in the Rochester Diabetic Neuropathy Study cohort. Neurology. 1997; 49:229–239.25. Toth C, Brussee V, Zochodne DW. Remote neurotrophic support of epidermal nerve fibres in experimental diabetes. Diabetologia. 2006; 49:1081–1088.26. Masaoka S, Lev-Ran A, Hill LR, Vakil G, Hon EH. Heart rate variability in diabetes: relationship to age and duration of the disease. Diabetes Care. 1985; 8:64–68.27. Ziegler D, Laux G, Dannehl K, Spüler M, Mühlen H, Mayer P, Gries FA. Assessment of cardiovascular autonomic function: age-related normal ranges and reproducibility of spectral analysis, vector analysis, and standard tests of heart rate variation and blood pressure responses. Diabet Med. 1992; 9:166–175.28. Kim YK, Lee JE, Kim YG, Kim DJ, Oh HY, Yang CW, Kim KW, Huh W. Cardiac autonomic neuropathy as a predictor of deterioration of the renal function in normoalbuminuric, normotensive patients with type 2 diabetes mellitus. J Korean Med Sci. 2009; 24:Suppl. S69–74.29. Chaturvedi N, Sjoelie AK, Porta M, Aldington SJ, Fuller JH, Songini M, Kohner EM; EURODIAB Prospective Complications Study. Markers of insulin resistance are strong risk factors for retinopathy incidence in type 1 diabetes. Diabetes Care. 2001; 24:284–289.30. Lee SH, Yang HK, Ha HS, Lee JH, Kwon HS, Park YM, Yim HW, Kang MI, Lee WC, Son HY, et al. Changes in metabolic health status over time and risk of developing type 2 diabetes: a Prospective Cohort Study. Medicine (Baltimore). 2015; 94:e1705.31. Moon S, Park JS, Ahn Y. The cut-off values of triglycerides and glucose index for metabolic syndrome in American and Korean adolescents. J Korean Med Sci. 2017; 32:427–433.32. Hosseini SM, Maleki A, Hosseininejad SM. Cardiac autonomic neuropathy and two insulin resistance indices. Enliven: Clin Cardiol Res. 2014; 1:006.33. Jeppesen J, Hansen TW, Rasmussen S, Ibsen H, Torp-Pedersen C, Madsbad S. Insulin resistance, the metabolic syndrome, and risk of incident cardiovascular disease: a population-based study. J Am Coll Cardiol. 2007; 49:2112–2119.34. Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Meigs JB, Bonadonna RC, Muggeo M. Insulin resistance as estimated by homeostasis model assessment predicts incident symptomatic cardiovascular disease in Caucasian subjects from the general population: the Bruneck study. Diabetes Care. 2007; 30:318–324.35. Bonora E, Formentini G, Calcaterra F, Lombardi S, Marini F, Zenari L, Saggiani F, Poli M, Perbellini S, Raffaelli A, et al. HOMA-estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabetes Care. 2002; 25:1135–1141.36. Bressler P, Bailey SR, Matsuda M, DeFronzo RA. Insulin resistance and coronary artery disease. Diabetologia. 1996; 39:1345–1350.37. Abbasi F, Reaven GM. Comparison of two methods using plasma triglyceride concentration as a surrogate estimate of insulin action in nondiabetic subjects: triglycerides × glucose versus triglyceride/high-density lipoprotein cholesterol. Metabolism. 2011; 60:1673–1676.38. Navarro-González D, Sánchez-Íñigo L, Pastrana-Delgado J, Fernández-Montero A, Martinez JA. Triglyceride-glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: the Vascular-Metabolic CUN cohort. Prev Med. 2016; 86:99–105.39. Wiggin TD, Sullivan KA, Pop-Busui R, Amato A, Sima AA, Feldman EL. Elevated triglycerides correlate with progression of diabetic neuropathy. Diabetes. 2009; 58:1634–1640.40. Lee KO, Nam JS, Ahn CW, Hong JM, Kim SM, Sunwoo IN, Moon JS, Na SJ, Choi YC. Insulin resistance is independently associated with peripheral and autonomic neuropathy in Korean type 2 diabetic patients. Acta Diabetol. 2012; 49:97–103.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment Strategies for Diabetic Neuropathy
- Relationship between Cardiovascular Autonomic Neuropathy and Diabetic Retinopathy in Patients with Non-Insulin Dependent Diabetes Mellitus
- Relationship between Peripheral Neuropathy and Cardiovascular Autonomic Neuropathy in Non-Insulin Dependent Diabetics
- Morphologic Changes in Autonomic Nerves in Diabetic Autonomic Neuropathy
- Diastolic Dysfunction of Left Ventricle by Cardiovascular Autonomic Neuropathy in Type 2 Diabetic Patients Without Cardiovascular Symptom