Korean Circ J.
2017 Sep;47(5):714-726. 10.4070/kcj.2017.0092.
Remote Ischemic Conditioning by Effluent Collected from a Novel Isolated Hindlimb Model Reduces Infarct Size in an Isolated Heart Model
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea. yubs@yonsei.ac.kr
- 2Cell Therapy and Tissue Engineering Center, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 3Animal Core, Central Research Laboratory, Yonsei University Wonju College of Medicine, Wonju, Korea.
- KMID: 2389604
- DOI: http://doi.org/10.4070/kcj.2017.0092
Abstract
- BACKGROUND AND OBJECTIVES
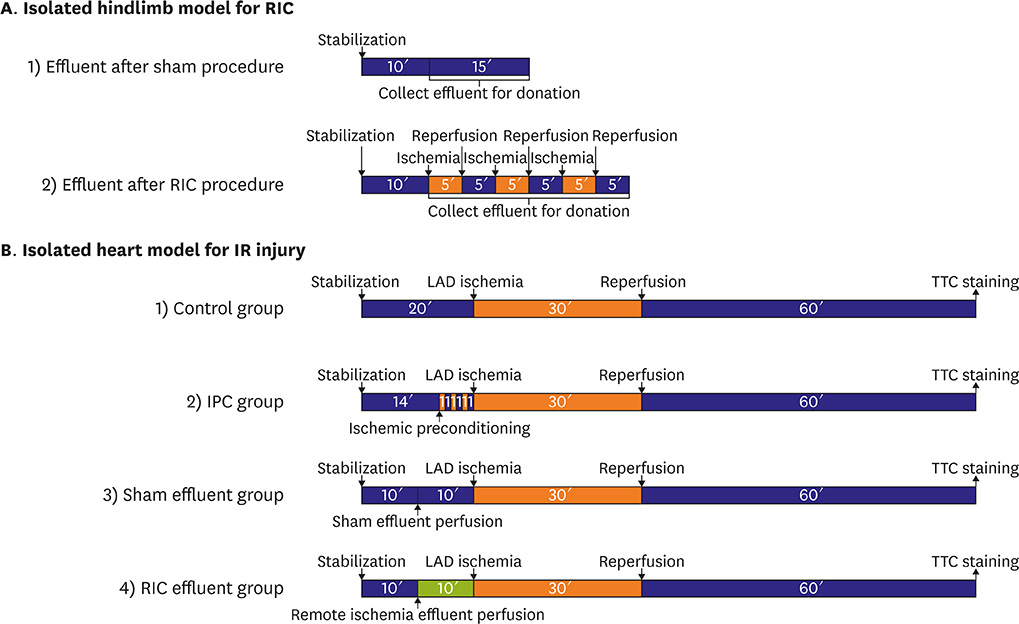
Experimental protocols for remote ischemic conditioning (RIC) utilize models in which a tourniquet is placed around the hindlimb or effluent is collected from an isolated heart. In analyzing the humoral factors that act as signal transducers in these models, sampled blood can be influenced by systemic responses, while the effluent from an isolated heart might differ from that of the hindlimb. Thus, we designed a new isolated hindlimb model for RIC and tested whether the effluent from this model could affect ischemia/reperfusion (IR) injury and if the reperfusion injury salvage kinase (RISK) and survivor activating factor enhancement (SAFE) pathways are involved in RIC.
MATERIALS AND METHODS
After positioning needles into the right iliac artery and vein of rats, Krebs-Henseleit buffer was perfused using a Langendorff apparatus, and effluent was collected. The RIC protocol consisted of 3 cycles of IR for 5 minutes. In the RIC effluent group, collected effluent was perfused in an isolated heart for 10 minutes before initiating IR injury.
RESULTS
Compared with the control group, the infarct area in the RIC effluent group was significantly smaller (31.2%±3.8% vs. 20.6%±1.8%, p<0.050), while phosphorylation of signal transducer and activation of transcription-3 (STAT-3) was significantly increased. However, there was a trend of increased phosphorylation of extracellular signal-regulated kinase (ERK) 1/2 in this group.
CONCLUSION
This is the first study to investigate the effect of effluent from a new isolated hindlimb model after RIC on IR injury in an isolated heart model. The RIC effluent was effective in reducing the IR injury, and the cardioprotective effect was associated with activation of the SAFE pathway.
Keyword
MeSH Terms
Figure
Reference
-
1. Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007; 357:1121–1135.2. Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986; 74:1124–1136.3. Zhao ZQ, Corvera JS, Halkos ME, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003; 285:H579–H588.4. Heusch G, Bøtker HE, Przyklenk K, Redington A, Yellon D. Remote ischemic conditioning. J Am Coll Cardiol. 2015; 65:177–195.5. Xin P, Zhu W, Li J, et al. Combined local ischemic postconditioning and remote perconditioning recapitulate cardioprotective effects of local ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2010; 298:H1819–H1831.6. Tamareille S, Mateus V, Ghaboura N, et al. RISK and SAFE signaling pathway interactions in remote limb ischemic perconditioning in combination with local ischemic postconditioning. Basic Res Cardiol. 2011; 106:1329–1339.7. Huffman LC, Koch SE, Butler KL. Coronary effluent from a preconditioned heart activates the JAK-STAT pathway and induces cardioprotection in a donor heart. Am J Physiol Heart Circ Physiol. 2008; 294:H257–H262.8. Breivik L, Helgeland E, Aarnes EK, Mrdalj J, Jonassen AK. Remote postconditioning by humoral factors in effluent from ischemic preconditioned rat hearts is mediated via PI3K/Akt-dependent cell-survival signaling at reperfusion. Basic Res Cardiol. 2011; 106:135–145.9. Hausenloy DJ, Yellon DM. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res. 2006; 70:240–253.10. Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res. 2004; 61:448–460.11. Lacerda L, Somers S, Opie LH, Lecour S. Ischaemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res. 2009; 84:201–208.12. Lecour S. Activation of the protective Survivor Activating Factor Enhancement (SAFE) pathway against reperfusion injury: does it go beyond the RISK pathway? J Mol Cell Cardiol. 2009; 47:32–40.13. Skyschally A, Gent S, Amanakis G, Schulte C, Kleinbongard P, Heusch G. Across-species transfer of protection by remote ischemic preconditioning with species-specific myocardial signal transduction by reperfusion injury salvage kinase and survival activating factor enhancement pathways. Circ Res. 2015; 117:279–288.14. Heidbreder M, Naumann A, Tempel K, Dominiak P, Dendorfer A. Remote vs. ischaemic preconditioning: the differential role of mitogen-activated protein kinase pathways. Cardiovasc Res. 2008; 78:108–115.15. Pickard JM, Davidson SM, Hausenloy DJ, Yellon DM. Co-dependence of the neural and humoral pathways in the mechanism of remote ischemic conditioning. Basic Res Cardiol. 2016; 111:50.16. Song BW, Hwang HJ, Seung M, Lee MH. Effect of hypoxic paracrine media on calcium-regulatory proteins in infarcted rat myocardium. Korean Circ J. 2014; 44:16–21.17. Li YW, Li YM, Hon Y, et al. AT1 receptor modulator attenuates the hypercholesterolemia-induced impairment of the myocardial ischemic post-conditioning benefits. Korean Circ J. 2017; 47:182–192.18. Ferrera R, Benhabbouche S, Bopassa JC, Li B, Ovize M. One hour reperfusion is enough to assess function and infarct size with TTC staining in Langendorff rat model. Cardiovasc Drugs Ther. 2009; 23:327–331.19. Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993; 87:893–899.20. Dickson EW, Lorbar M, Porcaro WA, et al. Rabbit heart can be “preconditioned” via transfer of coronary effluent. Am J Physiol. 1999; 277:H2451–H2457.21. Serejo FC, Rodrigues LF Jr, da Silva Tavares KC, de Carvalho AC, Nascimento JH. Cardioprotective properties of humoral factors released from rat hearts subject to ischemic preconditioning. J Cardiovasc Pharmacol. 2007; 49:214–220.22. Weinbrenner C, Nelles M, Herzog N, Sárváry L, Strasser RH. Remote preconditioning by infrarenal occlusion of the aorta protects the heart from infarction: a newly identified non-neuronal but PKC-dependent pathway. Cardiovasc Res. 2002; 55:590–601.23. Heinen NM, Pütz VE, Görgens JI, et al. Cardioprotection by remote ischemic preconditioning exhibits a signaling pattern different from local ischemic preconditioning. Shock. 2011; 36:45–53.24. Wong GT, Lu Y, Mei B, Xia Z, Irwin MG. Cardioprotection from remote preconditioning involves spinal opioid receptor activation. Life Sci. 2012; 91:860–865.25. Gross GJ, Baker JE, Moore J, Falck JR, Nithipatikom K. Abdominal surgical incision induces remote preconditioning of trauma (RPCT) via activation of bradykinin receptors (BK2R) and the cytochrome P450 epoxygenase pathway in canine hearts. Cardiovasc Drugs Ther. 2011; 25:517–522.26. Jones WK, Fan GC, Liao S, et al. Peripheral nociception associated with surgical incision elicits remote nonischemic cardioprotection via neurogenic activation of protein kinase C signaling. Circulation. 2009; 120:S1–S9.27. Gross ER, Hsu AK, Urban TJ, Mochly-Rosen D, Gross GJ. Nociceptive-induced myocardial remote conditioning is mediated by neuronal gamma protein kinase C. Basic Res Cardiol. 2013; 108:381.28. Sachdeva J, Dai W, Gerczuk PZ, Kloner RA. Combined remote perconditioning and postconditioning failed to attenuate infarct size and contractile dysfunction in a rat model of coronary artery occlusion. J Cardiovasc Pharmacol Ther. 2014; 19:567–573.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Sufentanil on Myocardial Function and Coronary Flow in an Isolated-Heart Rat Model
- Cardiodynamics and Infarct Size in Regional and Global Ischemic Isolated Heart Model: Comparison of 1 Hour and 2 Hours Reperfusion
- Cardioprotective Effect by Preconditioning with Calcium-free Solution
- Inhibiting Effect of Amiodarone to Protective Action against Ischemia/Reperfusion Injury Induced by Ischemic Preconditioning
- Effects of Humoral Factors Released from Ischemic-preconditioned Hearts on Survival of Pancreatic Cells Exposed to Hypoxia: An In vitro Study Using Neonatal Pigs