J Nutr Health.
2017 Jun;50(3):236-245. 10.4163/jnh.2017.50.3.236.
Effect of oral guava leaf extract administration on antioxidant and vasculoprotective activity in ovariectomized rats
- Affiliations
-
- 1Department of Food and Nutrition, Sookmyung Women's University, Seoul 04307, Korea. hskim@sookmyung.ac.kr
- KMID: 2386625
- DOI: http://doi.org/10.4163/jnh.2017.50.3.236
Abstract
- PURPOSE
The aim of this study was to assess the effects of guava leaf extract (GLE) supplementation on antioxidant enzyme activity and expression of endothelial nitric oxide synthase (eNOS) mRNA in ovariectomized rats.
METHODS
All animals were randomly assigned to four groups (n = 7 for each group): non-ovariectomized control (Sham), the ovariectomized control (OVX), ovariectomy + 150 mg/kg b.w. of GLE (OVX·GL), and ovariectomy + 300 mg/kg b.w. of GLE (OVX·GH). Treatment groups were administered GLE for 8 weeks every day.
RESULTS
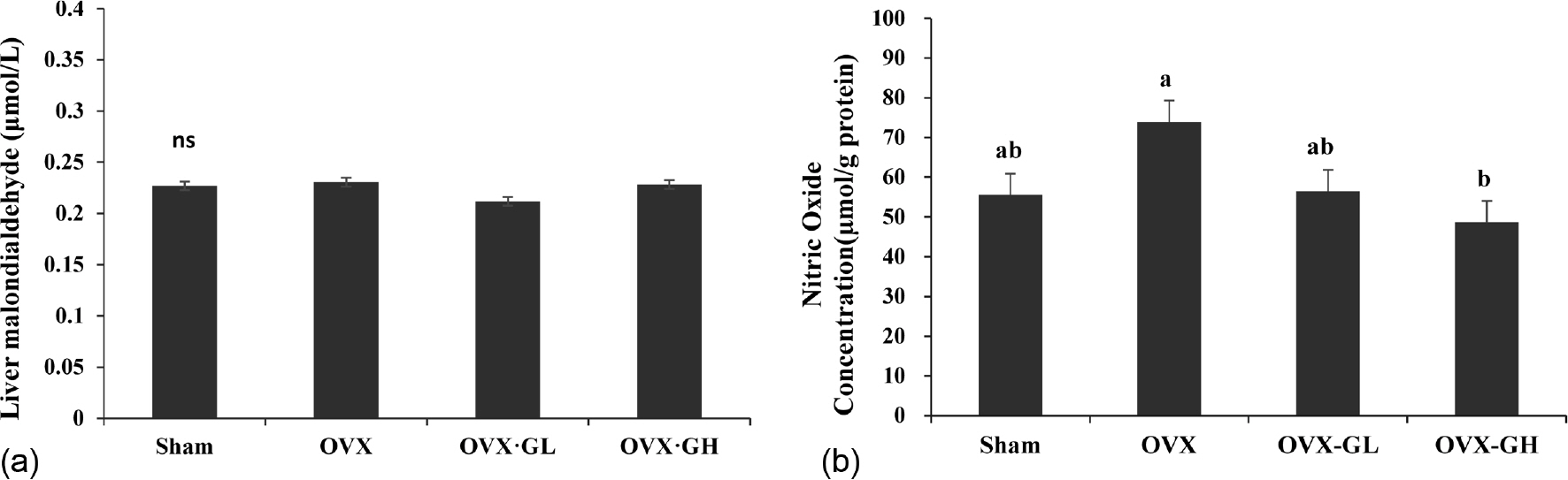
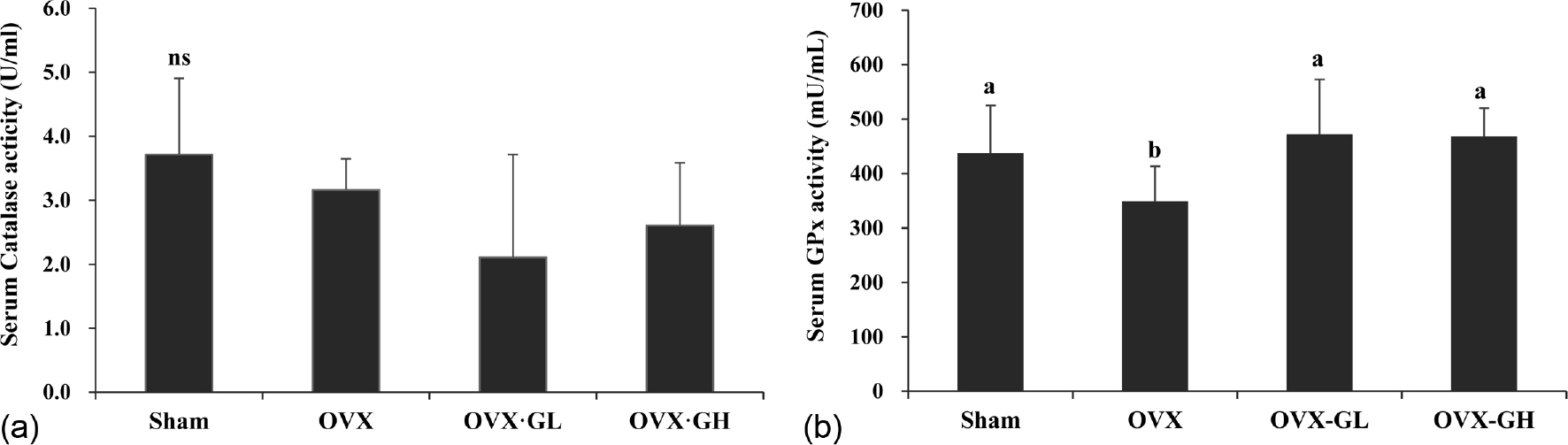
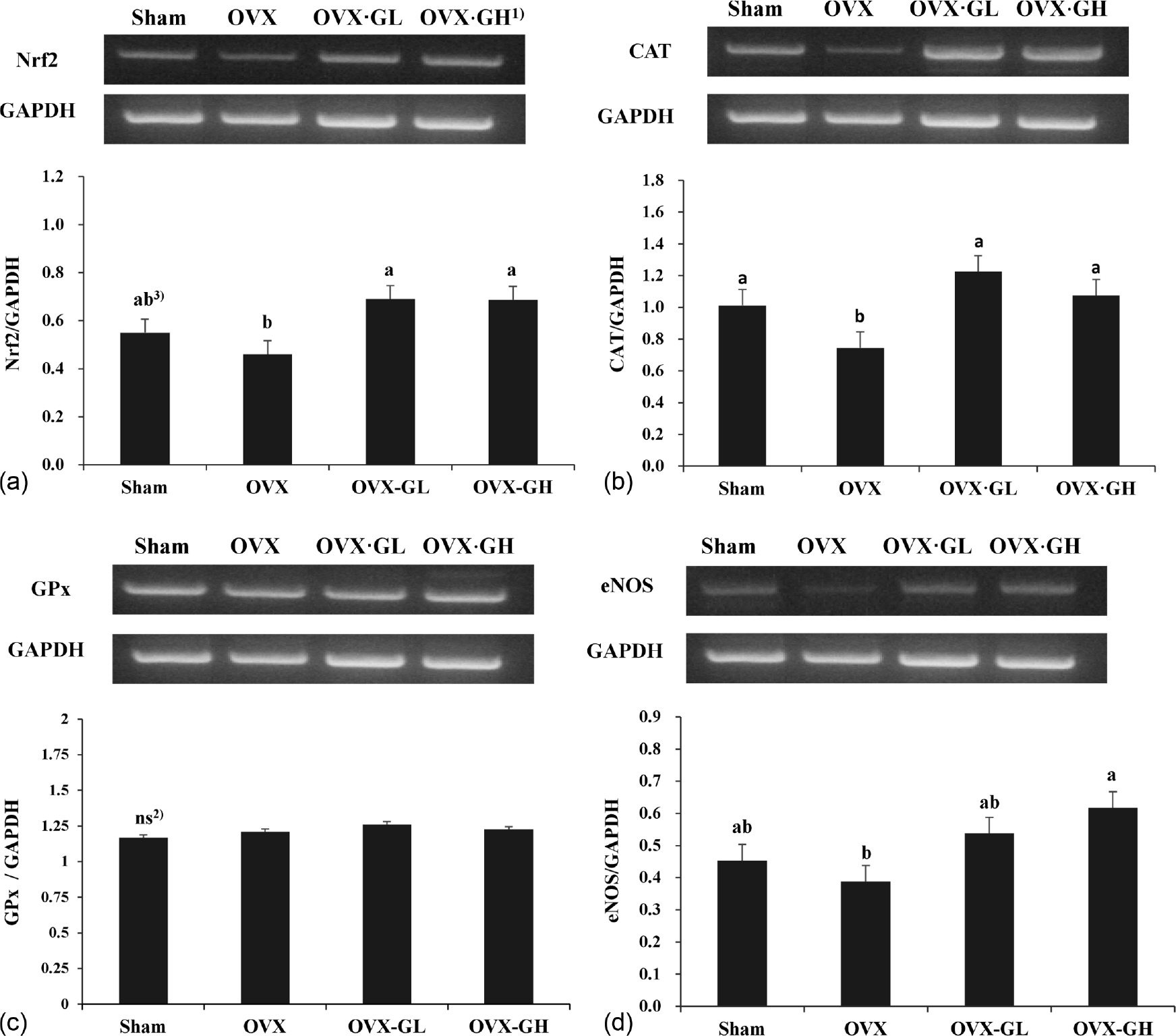
Body weight gain was significantly reduced in the OVX·GL group compared with the OVX group (p < 0.05). The level of serum 17β-estradiol (E2) was significantly lower in the OVX groups than the Sham group (p < 0.05). Serum triglyceride (TG) and HDL-cholesterol levels were not significantly different between all groups. However, serum total cholesterol (TC) level was significantly reduced in the OVX·GH group compared with the OVX group (p < 0.05). Serum free fatty acid (FFA) level and liver TG level were significantly reduced in both OVX·GL and OVX·GH groups compared with the OVX group (p < 0.05). Furthermore, serum glutathione peroxidase (GPx) activity was significantly elevated in the GLE groups (p < 0.05). The mRNA expression level of GPx was not affected by ovariectomy. However, administration of GLE resulted in significantly increased nuclear factor erythroid 2-related factor (Nrf2) and catalase (CAT) mRNA expression levels in the liver (p < 0.05). In addition, liver nitric oxide (NO) level was significantly reduced in the OVX·GH group compared with the OVX group (p < 0.05). Expression level of endothelial nitric oxide synthase (eNOS) was significantly elevated in the OVX·GH group compared with the OVX group (p < 0.05).
CONCLUSION
These results suggest that GLE could have protective effects in OVX rats by stimulating eNOS expression and improving the antioxidant defense system.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Salpeter SR, Walsh JM, Ormiston TM, Greyber E, Buckley NS, Salpeter EE. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab. 2006; 8(5):538–554.
Article2. Pasquali R, Casimirri F, Pascal G, Tortelli O, Morselli Labate A, Bertazzo D, Vicennati V, Gaddi A. Virgilio Menopause Health Group. Influence of menopause on blood cholesterol levels in women: the role of body composition, fat distribution and hormonal milieu. J Intern Med. 1997; 241(3):195–203.
Article3. Nasirzadeh M, Rasouli A. Pretreatment effect of alcoholic olive leaf extract on oxidative and antioxidative enzymes status in ovariectomized rats. Int J Womens Health Reprod Sci. 2016; 4(2):77–80.
Article4. Ramezani Tehrani F, Behboudi-Gandevani S, Ghasemi A, Azizi F. Association between serum concentrations of nitric oxide and transition to menopause. Acta Obstet Gynecol Scand. 2015; 94(7):708–714.
Article5. Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, Hsia J, Hulley S, Herd A, Khan S, Newby LK, Waters D, Vittinghoff E, Wenger N. HERS Research Group. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA. 2002; 288(1):49–57.6. Yoo JH, Liu Y, Kim HS. Hawthorn fruit extract elevates expression of Nrf2/HO-1 and improves lipid profiles in ovariectomized rats. Nutrients. 2016; 8(5):): E283.
Article7. Hwang HJ, Kang MS, Kim BK, Jung BM, Kim M. The effect of Opuntia humifusa seed extracts on platelet aggregation and serum lipid level in ovariectomized rats. J Life Sci. 2012; 22(12):1680–1687.
Article8. Patki G, Allam FH, Atrooz F, Dao AT, Solanki N, Chugh G, Asghar M, Jafri F, Bohat R, Alkadhi KA, Salim S. Grape powder intake prevents ovariectomy-induced anxiety-like behavior, memory impairment and high blood pressure in female Wistar rats. PLoS One. 2013; 8(9):): e74522.
Article9. Sevastre B, Taulescu M, Marcus I, Benedec D, Mocanu A, Han-ganu D. Protective effect of grape seed extract in experimental menopausal syndrome. Rev Med Chir Soc Med Nat Iasi. 2014; 118(3):860–865.10. Hsieh CL, Huang CN, Lin YC, Peng RY. Molecular action mechanism against apoptosis by aqueous extract from guava budding leaves elucidated with human umbilical vein endothelial cell (HUVEC) model. J Agric Food Chem. 2007; 55(21):8523–8533.
Article11. Irondi EA, Agboola SO, Oboh G, Boligon AA, Athayde ML, Shode FO. Guava leaves polyphenolics-rich extract inhibits vital enzymes implicated in gout and hypertension in vitro. J Intercult Ethnopharmacol. 2016; 5(2):122–130.
Article12. Díaz-de-Cerio E, Rodríguez-Nogales A, Algieri F, Romero M, Verardo V, Segura-Carretero A, Duarte J, Galvez J. The hypoglycemic effects of guava leaf (Psidium guajava L.) extract are associated with improving endothelial dysfunction in mice with diet-induced obesity. Food Res Int. 2017; 96:64–71.13. Yoshitomi H, Guo X, Liu T, Gao M. Guava leaf extracts alleviate fatty liver via expression of adiponectin receptors in SHRSP.Z-Leprfa/Izm rats. Nutr Metab (Lond). 2012; 9:13.
Article14. Deguchi Y, Miyazaki K. Anti-hyperglycemic and anti-hyperlipidemic effects of guava leaf extract. Nutr Metab (Lond). 2010; 7:9.
Article15. George L, Rajendran B, Manickam V, Ragothaman A, Sirajudeen K, Tamizhselvi R. Psidium guajava leaf extract modulates cyto-kine expression in lipopolysaccharide-activated primary mouse neutrophils thereby inhibiting NF-κB activity. Int J Biol Pharm Res. 2014; 5(11):870–875.16. Aebi H. Catalase in vitro. Methods Enzymol. 1984; 105:121–126.17. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957; 226(1):497–509.
Article18. Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978; 52:302–310.19. Das AS, Das D, Mukherjee M, Mukherjee S, Mitra C. Phytoestro-genic effects of black tea extract (Camellia sinensis) in an oophorectomized rat (Rattus norvegicus) model of osteoporosis. Life Sci. 2005; 77(24):3049–3057.
Article20. Misso ML, Jang C, Adams J, Tran J, Murata Y, Bell R, Boon WC, Simpson ER, Davis SR. Adipose aromatase gene expression is greater in older women and is unaffected by postmenopausal estrogen therapy. Menopause. 2005; 12(2):210–215.
Article21. Satoh K, Nonaka R, Ishikawa F, Ogata A, Nagai F. In vitro screening assay for detecting aromatase activity using rat ovarian micro-somes and estrone ELISA. Biol Pharm Bull. 2008; 31(3):357–362.
Article22. Monteiro R, Azevedo I, Calhau C. Modulation of aromatase activity by diet polyphenolic compounds. J Agric Food Chem. 2006; 54(10):3535–3540.
Article23. Wade GN, Gray JM, Bartness TJ. Gonadal influences on adiposity. Int J Obes. 1985; 9(Suppl 1):83–92.24. Serra MC, Ryan AS, Goldberg AP. Reduced LPL and subcutaneous lipid storage capacity are associated with metabolic syndrome in postmenopausal women with obesity. Obes Sci Pract. 2017; 3(1):106–114.
Article25. Mendelsohn ME, Karas RH. Estrogen and the blood vessel wall. Curr Opin Cardiol. 1994; 9(5):619–626.
Article26. Campos H, Wilson PW, Jiménez D, McNamara JR, Ordovas J, Schaefer EJ. Differences in apolipoproteins and low-density lipoprotein subfractions in postmenopausal women on and off estrogen therapy: results from the Framingham Offspring Study. Metabolism. 1990; 39(10):1033–1038.
Article27. Wu Q, Zhao Z, Sun H, Hao YL, Yan CD, Gu SL. Oestrogen changed cardiomyocyte contraction and β-adrenoceptor expression in rat hearts subjected to ischaemia-reperfusion. Exp Physiol. 2008; 93(9):1034–1043.
Article28. Guo X, Yoshitomi H, Gao M, Qin L, Duan Y, Sun W, Xu T, Xie P, Zhou J, Huang L, Liu T. Guava leaf extracts promote glucose metabolism in SHRSP.Z-Leprfa/Izm rats by improving insulin resistance in skeletal muscle. BMC Complement Altern Med. 2013; 13:52.
Article29. Motoshima H, Wu X, Sinha MK, Hardy VE, Rosato EL, Barbot DJ, Rosato FE, Goldstein BJ. Differential regulation of adiponectin secretion from cultured human omental and subcutaneous adipocytes: effects of insulin and rosiglitazone. J Clin Endocrinol Metab. 2002; 87(12):5662–5667.
Article30. Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, Ohtsuka-Kowatari N, Kumagai K, Sakamoto K, Kobayashi M, Yamauchi T, Ueki K, Oishi Y, Nishimura S, Manabe I, Hashimoto H, Ohnishi Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Nagai R, Kadowaki T. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem. 2006; 281(36):26602–26614.
Article31. Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend Med. 2009; 6(Suppl 1):60–75.
Article32. Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond). 2011; 120(9):357–375.
Article33. Effendy NM, Shuid AN. Time and dose-dependent effects of Labi-sia pumila on bone oxidative status of postmenopausal osteoporosis rat model. Nutrients. 2014; 6(8):3288–3302.
Article34. Dong J, Sulik KK, Chen SY. Nrf2-mediated transcriptional induction of antioxidant response in mouse embryos exposed to ethanol in vivo: implications for the prevention of fetal alcohol spectrum disorders. Antioxid Redox Signal. 2008; 10(12):2023–2033.35. Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007; 47(1):89–116.
Article36. Dash DK, Yeligar VC, Nayak SS, Ghosh T, Rajalingam R, Sengupta P, Maiti BC, Maity TK. Evaluation of hepatoprotective and antioxidant activity of Ichnocarpus frutescens (Linn.) R.Br. on paracetamol-induced hepatotoxicity in rats. Trop J Pharm Res. 2007; 6(3):755–65.
Article37. Thaipong K, Boonprakob U, Cisneros-Zevallos L, Byrne DH. Hydrophilic and lipophilic antioxidant activities of guava fruits. Southeast Asian J Trop Med Public Health. 2005; 36(Suppl 4):254–257.38. Kireev RA, Tresguerres AF, Vara E, Ariznavarreta C, Tresguerres JA. Effect of chronic treatments with GH, melatonin, estrogens, and phytoestrogens on oxidative stress parameters in liver from aged female rats. Biogerontology. 2007; 8(5):469–482.
Article39. Lee YM, Cheng PY, Hong SF, Chen SY, Lam KK, Sheu JR, Yen MH. Oxidative stress induces vascular heme oxygenase-1 expression in ovariectomized rats. Free Radic Biol Med. 2005; 39(1):108–117.
Article40. Kröncke KD, Fehsel K, Kolb-Bachofen V. Nitric oxide: cytotoxicity versus cytoprotection–how, why, when, and where? Nitric Oxide. 1997; 1(2):107–120.41. Laurent M, Lepoivre M, Tenu JP. Kinetic modelling of the nitric oxide gradient generated in vitro by adherent cells expressing inducible nitric oxide synthase. Biochem J. 1996; 314(Pt 1):109–113.
Article42. Cau SB, Carneiro FS, Tostes RC. Differential modulation of nitric oxide synthases in aging: therapeutic opportunities. Front Physiol. 2012; 3:218.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The antihypertensive effect of ethyl acetate extract of radish leaves in spontaneously hypertensive rats
- Lotus leaf alleviates hyperglycemia and dyslipidemia in animal model of diabetes mellitus
- Exploration of the anti‑diabetic potential of hydro‑ethanolic leaf extract of Koenigia polystachya L.: an edible wild plant from Northeastern India
- Postprandial hypoglycemic effect of mulberry leaf in Goto-Kakizaki rats and counterpart control Wistar rats
- Effects of Boron Supplementation on Lipid Profiles and Antioxidant Capacities in the Ovariectomized Rats