Nutr Res Pract.
2012 Aug;6(4):308-314.
The antihypertensive effect of ethyl acetate extract of radish leaves in spontaneously hypertensive rats
- Affiliations
-
- 1Department of Foods and Nutrition, Kookmin University, 861-1, Chongneung-dong, Sungbuk-gu, Seoul 136-702, Korea. cmoon@kookmin.ac.kr
- 2Premedical course, College of Medicine, Dankook University, Cheonan 330-714, Korea.
- 3Department of Functional Food and Nutrition Division, Rural National Academy of Agricultural Science, Suwon 441-707, Korea.
Abstract
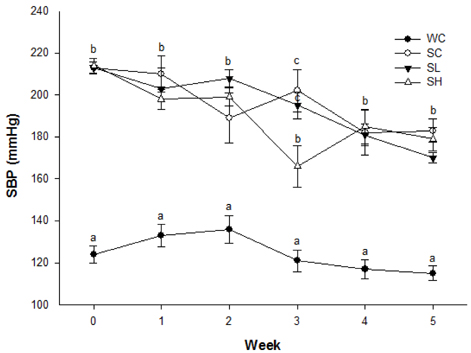
- Radish (Raphanus sativus L.) is a cruciferous vegetable, and its leaves have antioxidant and anticancer properties. This study was conducted to evaluate the effects of ethyl acetate extracts from radish leaves on hypertension in 11-week-old spontaneously hypertensive rats (SHRs). The SHRs were randomly divided into 3 groups of 6 rats each on the basis of initial systolic blood pressure (SBP) and were treated with oral administration of radish leaf extract (0, 30, or 90 mg/kg body weight [bw], respectively) for 5 weeks. Six Wistar rats were used as normotensive controls. The amount of the radish leaf extract had no effect on body weight. The SBP of the SHRs showed a decreasing trend with the consumption of the radish leaf extract. In the third week, the SBP of the group fed 90 mg extract/kg bw reduced from 214 mmHg to 166 mmHg and was significantly lower than that of the normotensive and hypertensive controls. The extract did not show a significant effect on the angiotensin-converting enzyme (ACE) activity in the serum, kidney, and lung. The extract increased the concentration of NO in serum and the activities of antioxidant enzymes such as glutathione peroxidase and catalase in red blood cells (RBCs). The serum concentrations of Na+ and K+ were not significantly different between all groups. However, the fecal concentrations of Na+ and K+ increased; the fecal concentrations of Na+ and K+ for the normotensive and hypertensive controls were not different. Urinary excretion of Na+ was higher in the normotensive Wistar rats than in the SHRs, while that of K+ was not significantly different. These findings indicate that consumption of radish leaves might have had antihypertensive effects in SHRs by increasing the serum concentration of NO and fecal concentration of Na+ and enhancing antioxidant activities.
Keyword
MeSH Terms
Figure
Reference
-
1. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute. National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003. 42:1206–1252.
Article2. Chobanian AV. Mixed messages on blood pressure goals. Hypertension. 2011. 57:1039–1040.
Article3. Mennen LI, Sapinho D, de Bree A, Arnault N, Bertrais S, Galan P, Hercberg S. Consumption of foods rich in flavonoids is related to a decreased cardiovascular risk in apparently healthy French women. J Nutr. 2004. 134:923–926.
Article4. Lakshmi SV, Padmaja G, Kuppusamy P, Kutala VK. Oxidative stress in cardiovascular disease. Indian J Biochem Biophys. 2009. 46:421–440.5. Ignarro LJ. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Pharmacol Toxicol. 1990. 30:535–560.
Article6. Taddei S, Virdis A, Mattei P, Ghiadoni L, Fasolo CB, Sudano I, Salvetti A. Hypertension causes premature aging of endothelial function in humans. Hypertension. 1997. 29:736–743.
Article7. Klahr S. The role of nitric oxide in hypertension and renal disease progression. Nephrol Dial Transplant. 2001. 16:Suppl 1. 60–62.
Article8. Carr A, Frei B. The role of natural antioxidants in preserving the biological activity of endothelium-derived nitric oxide. Free Radic Biol Med. 2000. 28:1806–1814.
Article9. Zalba G, Beaumont J, San José G, Fortuño A, Fortuño MA, Díez J. Vascular oxidant stress: molecular mechanisms and pathophysiological implications. J Physiol Biochem. 2000. 56:57–64.
Article10. Erdös EG, Skidgel RA. The angiotensin I-converting enzyme. Lab Invest. 1987. 56:345–348.11. Iwatsubo H, Nagano M, Sakai T, Kumamoto K, Morita R, Higaki J, Ogihara T, Hata T. Converting enzyme inhibitor improves forearm reactive hyperemia in essential hypertension. Hypertension. 1997. 29:286–290.
Article12. Schiffrin EL, Deng LY. Comparison of effects of angiotensin I-converting enzyme inhibition and beta-blockade for 2 years on function of small arteries from hypertensive patients. Hypertension. 1995. 25:699–703.
Article13. Wing LM, Reid CM, Ryan P, Beilin LJ, Brown MA, Jennings GL, Johnston CI, McNeil JJ, Macdonald GJ, Marley JE, Morgan TO, West MJ. Second Australian National Blood Pressure Study Group. A comparison of outcomes with angiotensin-converting--enzyme inhibitors and diuretics for hypertension in the elderly. N Engl J Med. 2003. 348:583–592.
Article14. DiBianco R. Angiotensin converting enzyme inhibition. Unique and effective therapy for hypertension and congestive heart failure. Postgrad Med. 1985. 78:229–241. 244247–248.15. Buijsse B, Feskens EJ, Kok FJ, Kromhout D. Cocoa intake, blood pressure, and cardiovascular mortality: the Zutphen Elderly Study. Arch Intern Med. 2006. 166:411–417.
Article16. Heiss C, Kleinbongard P, Dejam A, Perré S, Schroeter H, Sies H, Kelm M. Acute consumption of flavanol-rich cocoa and the reversal of endothelial dysfunction in smokers. J Am Coll Cardiol. 2005. 46:1276–1283.
Article17. Kalea AZ, Clark K, Schuschke DA, Kristo AS, Klimis-Zacas DJ. Dietary enrichment with wild blueberries (Vaccinium angustifolium) affects the vascular reactivity in the aorta of young spontaneously hypertensive rats. J Nutr Biochem. 2010. 21:14–22.
Article18. Grassi D, Desideri G, Necozione S, Lippi C, Casale R, Properzi G, Blumberg JB, Ferri C. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J Nutr. 2008. 138:1671–1676.
Article19. Peng N, Clark JT, Prasain J, Kim H, White CR, Wyss JM. Antihypertensive and cognitive effects of grape polyphenols in estrogen-depleted, female, spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2005. 289:R771–R775.
Article20. Diebolt M, Bucher B, Andriantsitohaina R. Wine polyphenols decrease blood pressure, improve NO vasodilatation, and induce gene expression. Hypertension. 2001. 38:159–165.
Article21. Duffy SJ, Keaney JF Jr, Holbrook M, Gokce N, Swerdloff PL, Frei B, Vita JA. Short- and long-term black tea consumption reverses endothelial dysfunction in patients with coronary artery disease. Circulation. 2001. 104:151–156.
Article22. Lugasi A, Dworschák E, Blázovics A, Kéry Á. Antioxidant and free radical scavenging properties of squeezed juice from black radish (Raphanus sativus l. var niger) root. Phytother Res. 1998. 12:502–506.
Article23. Chuanphongpanich S, Phanichphant S, Bhuddasukh D, Suttajit M, Sirithunyalug B. Bioactive glucosinolates and antioxidant properties of broccoli seeds cultivated in Thailand. Warasan Songkhla Nakharin. 2006. 28:55–61.24. Ippoushi K, Takeuchi A, Ito H, Horie H, Azuma K. Antioxidative effects of daikon sprout (Raphanus sativus L.) and ginger (Zingiber officinale Roscoe) in rats. Food Chem. 2007. 102:237–242.
Article25. Kim BR, Park JH, Kim SH, Cho KJ, Chang MJ. Antihypertensive properties of dried radish leaves powder in spontaneously hypertensive rats. Korean J Nutr. 2010. 43:561–569.
Article26. Muramatsu K, Fukuyo M, Hara Y. Effect of green tea catechins on plasma cholesterol level in cholesterol-fed rats. J Nutr Sci Vitaminol (Tokyo). 1986. 32:613–622.
Article27. Hurst PL, Lovell-Smith CJ. Optimized assay for serum angiotensin-converting enzyme activity. Clin Chem. 1981. 27:2048–2052.
Article28. Liu X, Miller MJ, Joshi MS, Sadowska-Krowicka H, Clark DA, Lancaster JR Jr. Diffusion-limited reaction of free nitric oxide with erythrocytes. J Biol Chem. 1998. 273:18709–18713.
Article29. Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001. 5:62–71.
Article30. Beers RF Jr, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952. 195:133–140.
Article31. Draper HH, Squires EJ, Mahmoodi H, Wu J, Agarwal S, Hadley M. A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free Radic Biol Med. 1993. 15:353–363.
Article32. Wolin MS. Interactions of oxidants with vascular signaling systems. Arterioscler Thromb Vasc Biol. 2000. 20:1430–1442.
Article33. Kumar KV, Das UN. Are free radicals involved in the pathobiology of human essential hypertension? Free Radic Res Commun. 1993. 19:59–66.
Article34. Suzuki H, Swei A, Zweifach BW, Schmid-Schönbein GW. In vivo evidence for microvascular oxidative stress in spontaneously hypertensive rats. Hydroethidine microfluorography. Hypertension. 1995. 25:1083–1089.
Article35. Shieh FK, Kotlyar E, Sam F. Aldosterone and cardiovascular remodelling: focus on myocardial failure. J Renin Angiotensin Aldosterone Syst. 2004. 5:3–13.
Article36. Zhang CY, Tan BK. Hypotensive activity of aqueous extract of Andrographis paniculata in rats. Clin Exp Pharmacol Physiol. 1996. 23:675–678.
Article37. Raij L. Workshop: hypertension and cardiovascular risk factors: role of the angiotensin II-nitric oxide interaction. Hypertension. 2001. 37:767–773.
Article38. Ichiki T, Usui M, Kato M, Funakoshi Y, Ito K, Egashira K, Takeshita A. Downregulation of angiotensin II type 1 receptor gene transcription by nitric oxide. Hypertension. 1998. 31:342–348.
Article39. Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994. 298:249–258.
Article40. Takemoto M, Egashira K, Usui M, Numaguchi K, Tomita H, Tsutsui H, Shimokawa H, Sueishi K, Takeshita A. Important role of tissue angiotensin-converting enzyme activity in the pathogenesis of coronary vascular and myocardial structural changes induced by long-term blockade of nitric oxide synthesis in rats. J Clin Invest. 1997. 99:278–287.
Article41. Laragh JH, Baer L, Brunner HR, Buhler FR, Sealey JE, Vaughan ED Jr. Renin, angiotensin and aldosterone system in pathogenesis and management of hypertensive vascular disease. Am J Med. 1972. 52:633–652.
Article42. Lee YK, Sung CJ, Choi MK. A study on sodium and potassium balance of college women in Seoul. Korean J Community Nutr. 1999. 4:375–381.43. Khaw KT, Barrett-Connor E. Dietary potassium and stroke-associated mortality. A 12-year prospective population study. N Engl J Med. 1987. 316:235–240.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antihypertensive Properties of Dried Radish Leaves Powder in Spontaneously Hypertensive Rats
- A Comparative Study of Dichloromethane and Ethyl Acetate Root Extracts of Celosia trigyna: Phytochemical and Wound Healing Effect Analyses
- A Study on Recovery from Smell Dysfunction Induced by 3-Methylindole in Rats
- Effect of Artemisia Capillaris Extract on the Growth of Food-Borne Pathogens
- Antimicrobial Effect of Pulsatilla Koreana Extracts on Food-Borne Pathogens