Yonsei Med J.
2014 Mar;55(2):387-394.
Effects of Stress on Mouse beta-Defensin-3 Expression in the Upper Digestive Mucosa
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, Jichi Medical University, Tochigi, Japan.
- 2Department of Environmental Pathology and Research Institute of Salivary Gland Health Medicine, Kanagawa Dental University, Kanagawa, Japan. eternal.wish@jichi.ac.jp
Abstract
- PURPOSE
Gastrointestinal integrity and immune surveillance are affected by stress. Stress also adversely affects mucosal barrier function. beta-defensins constitute an integral component of the innate immune system as antimicrobial peptides, serving as the first line of defense against microbial pathogens at the epithelial surfaces of the upper digestive mucosa. The primary objective of this study was to determine the effects of stress on the expression profile of mouse beta-defensin-3 in the upper digestive mucosa of mice with diabetes.
MATERIALS AND METHODS
We established a mouse model of restraint stress by using NSY/Hos mice with type 2 diabetes mellitus. We used real-time polymerase chain reaction, in situ hybridization, and immunohistochemistry to investigate the effects of stress and glucocorticoid administration on mouse beta-defensin-3 expression in the upper digestive mucosa of the gingiva, esophagus, and stomach.
RESULTS
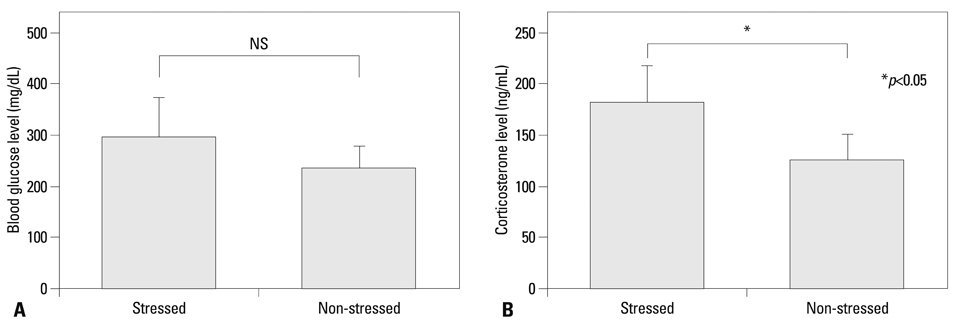
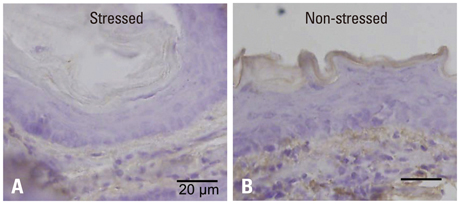
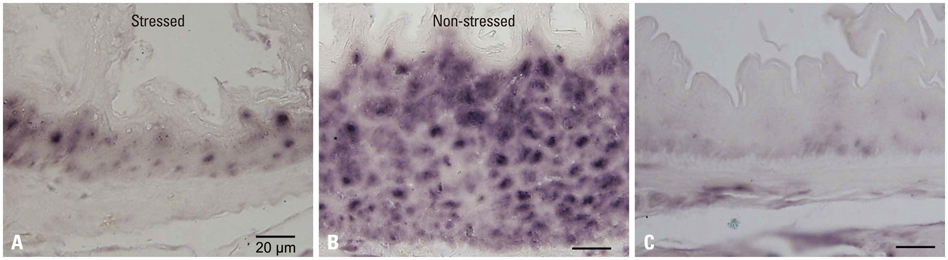
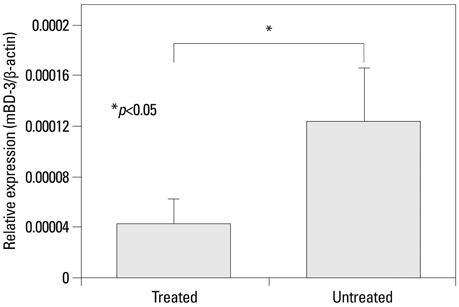
Mouse beta-defensin-3 mRNA expression was higher in the esophagus than in the gingiva or stomach (p<0.05). In the esophagus, mouse beta-defensin-3 mRNA expression was lower in stressed mice than in non-stressed mice (p<0.05). Furthermore, immunoreactivity to mouse beta-defensin-3 protein was lower in the esophagus of stressed mice than non-stressed mice, consistent with the results of mRNA expression analysis. Systemic glucocorticoid administration also downregulated esophageal mouse beta-defensin-3 mRNA expression.
CONCLUSION
Our novel findings show that stress decreases mouse beta-defensin-3 expression in the esophagus of mice with diabetes, possibly due to increased endogenous glucocorticoid production. It appears to be highly likely that stress management may normalize mucosal antimicrobial defenses in patients with diabetes.
Keyword
MeSH Terms
Figure
Reference
-
1. Meddings JB, Swain MG. Environmental stress-induced gastrointestinal permeability is mediated by endogenous glucocorticoids in the rat. Gastroenterology. 2000; 119:1019–1028.
Article2. Mawdsley JE, Rampton DS. Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut. 2005; 54:1481–1491.
Article3. Glaser R. Stress-associated immune dysregulation and its importance for human health: a personal history of psychoneuroimmunology. Brain Behav Immun. 2005; 19:3–11.
Article4. Marucha PT, Kiecolt-Glaser JK, Favagehi M. Mucosal wound healing is impaired by examination stress. Psychosom Med. 1998; 60:362–365.
Article5. Ebrecht M, Hextall J, Kirtley LG, Taylor A, Dyson M, Weinman J. Perceived stress and cortisol levels predict speed of wound healing in healthy male adults. Psychoneuroendocrinology. 2004; 29:798–809.
Article6. Horan MP, Quan N, Subramanian SV, Strauch AR, Gajendrareddy PK, Marucha PT. Impaired wound contraction and delayed myofibroblast differentiation in restraint-stressed mice. Brain Behav Immun. 2005; 19:207–216.
Article7. Gudeman SK, Wheeler CB, Miller JD, Halloran LG, Becker DP. Gastric secretory and mucosal injury response to severe head trauma. Neurosurgery. 1983; 12:175–179.
Article8. Kiecolt-Glaser JK, Marucha PT, Malarkey WB, Mercado AM, Glaser R. Slowing of wound healing by psychological stress. Lancet. 1995; 346:1194–1196.
Article9. Martin-Ezquerra G, Man MQ, Hupe M, Rodriguez-Martin M, Youm JK, Trullas C, et al. Psychological stress regulates antimicrobial peptide expression by both glucocorticoid and β-adrenergic mechanisms. Eur J Dermatol. 2011; 21:Suppl 2. 48–51.
Article10. Choi EH, Brown BE, Crumrine D, Chang S, Man MQ, Elias PM, et al. Mechanisms by which psychologic stress alters cutaneous permeability barrier homeostasis and stratum corneum integrity. J Invest Dermatol. 2005; 124:587–595.
Article11. Aberg KM, Radek KA, Choi EH, Kim DK, Demerjian M, Hupe M, et al. Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. J Clin Invest. 2007; 117:3339–3349.
Article12. Choi EH, Demerjian M, Crumrine D, Brown BE, Mauro T, Elias PM, et al. Glucocorticoid blockade reverses psychological stress-induced abnormalities in epidermal structure and function. Am J Physiol Regul Integr Comp Physiol. 2006; 291:R1657–R1662.
Article13. Denda M, Tsuchiya T, Elias PM, Feingold KR. Stress alters cutaneous permeability barrier homeostasis. Am J Physiol Regul Integr Comp Physiol. 2000; 278:R367–R372.
Article14. Goodson WH 3rd, Hung TK. Studies of wound healing in experimental diabetes mellitus. J Surg Res. 1977; 22:221–227.
Article15. Bader MS. Diabetic foot infection. Am Fam Physician. 2008; 78:71–79.16. Diamond G, Zasloff M, Eck H, Brasseur M, Maloy WL, Bevins CL. Tracheal antimicrobial peptide, a cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc Natl Acad Sci U S A. 1991; 88:3952–3956.
Article17. Lehrer RI, Ganz T. Defensins of vertebrate animals. Curr Opin Immunol. 2002; 14:96–102.
Article18. Diamond G, Beckloff N, Ryan LK. Host defense peptides in the oral cavity and the lung: similarities and differences. J Dent Res. 2008; 87:915–927.
Article19. Diamond G, Ryan L. Beta-defensins: what are they really doing in the oral cavity? Oral Dis. 2011; 17:628–635.
Article20. Bals R, Wang X, Meegalla RL, Wattler S, Weiner DJ, Nehls MC, et al. Mouse beta-defensin 3 is an inducible antimicrobial peptide expressed in the epithelia of multiple organs. Infect Immun. 1999; 67:3542–3547.
Article21. Abiko Y, Saitoh M, Nishimura M, Yamazaki M, Sawamura D, Kaku T. Role of beta-defensins in oral epithelial health and disease. Med Mol Morphol. 2007; 40:179–184.
Article22. Denda M, Tsuchiya T, Hosoi J, Koyama J. Immobilization-induced and crowded environment-induced stress delay barrier recovery in murine skin. Br J Dermatol. 1998; 138:780–785.
Article23. Altemus M, Rao B, Dhabhar FS, Ding W, Granstein RD. Stress-induced changes in skin barrier function in healthy women. J Invest Dermatol. 2001; 117:309–317.
Article24. Garg A, Chren MM, Sands LP, Matsui MS, Marenus KD, Feingold KR, et al. Psychological stress perturbs epidermal permeability barrier homeostasis: implications for the pathogenesis of stress-associated skin disorders. Arch Dermatol. 2001; 137:53–59.25. Anai M, Funaki M, Ogihara T, Kanda A, Onishi Y, Sakoda H, et al. Enhanced insulin-stimulated activation of phosphatidylinositol 3-kinase in the liver of high-fat-fed rats. Diabetes. 1999; 48:158–169.
Article26. Nojima K, Ikegami H, Fujisawa T, Ueda H, Babaya N, Itoi-Babaya M, et al. Food hardness as environmental factor in development of type 2 diabetes. Diabetes Res Clin Pract. 2006; 74:1–7.
Article27. Nakajima K, Hamada N, Takahashi Y, Sasaguri K, Tsukinoki K, Umemoto T, et al. Restraint stress enhances alveolar bone loss in an experimental rat model. J Periodontal Res. 2006; 41:527–534.
Article28. Saruta J, Iida M, Kondo Y, To M, Hayashi T, Hori M, et al. Chronic stress induces neurotrophin-3 in rat submandibular gland. Yonsei Med J. 2012; 53:1085–1092.
Article29. Kao JS, Fluhr JW, Man MQ, Fowler AJ, Hachem JP, Crumrine D, et al. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. J Invest Dermatol. 2003; 120:456–464.
Article30. To M, Kamata Y, Saruta J, Shimizu T, Sato T, Kondo Y, et al. Induction of β-Defensin Expression by Porphyromonas gingivalis-Infected Human Gingival Graft Transplanted in nu/nu Mouse Subdermis. Acta Histochem Cytochem. 2013; 46:25–34.
Article31. Kondo Y, Saruta J, To M, Shiiki N, Sato C, Tsukinoki K. Expression and Role of the BDNF Receptor-TrkB in Rat Adrenal Gland under Acute Immobilization Stress. Acta Histochem Cytochem. 2010; 43:139–147.
Article32. Saruta J, Fujino K, To M, Tsukinoki K. Expression and localization of brain-derived neurotrophic factor (BDNF) mRNA and protein in human submandibular gland. Acta Histochem Cytochem. 2012; 45:211–218.
Article33. Hosaka Y, Koslowski M, Nuding S, Wang G, Schlee M, Schäfer C, et al. Antimicrobial host defense in the upper gastrointestinal tract. Eur J Gastroenterol Hepatol. 2008; 20:1151–1158.
Article34. Shibata M, Yasuda B. New experimental congenital diabetic mice (NSY mice). Tohoku J Exp Med. 1980; 130:139–142.35. Takeda S, Sato N, Uchio-Yamada K, Sawada K, Kunieda T, Takeuchi D, et al. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci U S A. 2010; 107:7036–7041.
Article36. Saruta J, Kondo Y, Sato C, Shiiki N, Tsukinoki K, Sato S. Salivary glands as the source of plasma brain-derived neurotrophic factor in stressed rats engaged in biting behavior. Stress. 2010; 13:238–247.
Article37. Abiko Y, Suraweera AK, Nishimura M, Arakawa T, Takuma T, Mizoguchi I, et al. Differential expression of human beta-defensin 2 in keratinized and non-keratinized oral epithelial lesions; immunohistochemistry and in situ hybridization. Virchows Arch. 2001; 438:248–253.
Article38. Dale BA, Kimball JR, Krisanaprakornkit S, Roberts F, Robinovitch M, O'Neal R, et al. Localized antimicrobial peptide expression in human gingiva. J Periodontal Res. 2001; 36:285–294.
Article39. Gomez-Merino D, Drogou C, Chennaoui M, Tiollier E, Mathieu J, Guezennec CY. Effects of combined stress during intense training on cellular immunity, hormones and respiratory infections. Neuroimmunomodulation. 2005; 12:164–172.
Article40. Kiank C, Holtfreter B, Starke A, Mundt A, Wilke C, Schütt C. Stress susceptibility predicts the severity of immune depression and the failure to combat bacterial infections in chronically stressed mice. Brain Behav Immun. 2006; 20:359–368.
Article41. Cacioppo JT, Kiecolt-Glaser JK, Malarkey WB, Laskowski BF, Rozlog LA, Poehlmann KM, et al. Autonomic and glucocorticoid associations with the steady-state expression of latent Epstein-Barr virus. Horm Behav. 2002; 42:32–41.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Antimicrobial Defensin Peptides of the Human Nasal Mucosa
- Expression of beta Defensins in the Human Middle Ear Mucosa
- The Expression of beta-defensin 1 in Various Skin Tumors
- The Expression of Human beta-defensin 2 in Helicobacter pylori Infection
- Human beta-defensin 2 is induced by interleukin-1b in the cornealepithelial cells