Ann Rehabil Med.
2017 Feb;41(1):1-8. 10.5535/arm.2017.41.1.1.
Neurocognitive Dysfunction According to Hypoperfusion Territory in Patients With Moyamoya Disease
- Affiliations
-
- 1Department of Rehabilitation Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. mhchun@amc.seoul.kr
- KMID: 2383671
- DOI: http://doi.org/10.5535/arm.2017.41.1.1
Abstract
OBJECTIVE
To demonstrate the prevalence of cerebral hypoperfusion without focal cerebral lesions in patients with Moyamoya disease (MMD), and the relationship between areas of hypoperfusion and cognitive impairment.
METHODS
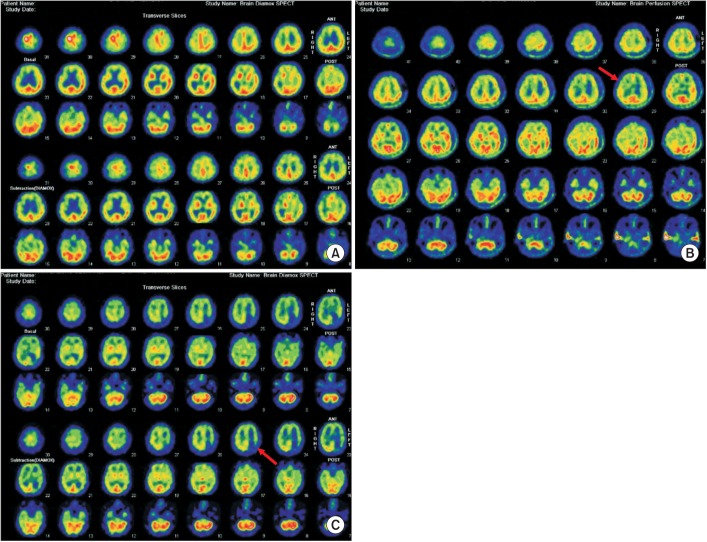
Twenty-six MMD patients were included. Patients were categorized according to the presence/absence of hypoperfusion in the frontal, parietal, temporal, and occipital lobes on brain single-photon-emission computed tomography (SPECT) after acetazolamide challenge. Computerized neuropsychological test (CNT) results were compared between groups.
RESULTS
Only 3 patients showed normal cerebral perfusion. Baseline characteristics were similar between groups. Patients with frontal lobe hypoperfusion showed lower scores in visual continuous performance test (CPT), auditory CPT, forward digit span test, backward digit span test, verbal learning test, and trail-making test. Patients with parietal lobe hypoperfusion showed lower backward digit span test, visual learning test, and trail-making test scores. Related to temporal and occipital lobes, there were no significant differences in CNT results between the hypoperfusion and normal groups.
CONCLUSION
MMD patients without focal cerebral lesion frequently exhibit cerebral hypoperfusion. MMD patients with frontal and parietal hypoperfusion had abnormal CNT profiles, similar to those with frontal and parietal lesions. It is suggested that the hypoperfusion territory on brain SPECT without focal lesion may affect the characteristics of neurocognitive dysfunction in MMD patients.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Value of the Frontal Assessment Battery Tool for Assessing the Frontal Lobe Function in Stroke Patients
Mihyang Han, Da-Ye Kim, Ja-Ho Leigh, Min-Wook Kim
Ann Rehabil Med. 2020;44(4):261-272. doi: 10.5535/arm.19111.
Reference
-
1. Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med. 2009; 360:1226–1237. PMID: 19297575.
Article2. Takeuchi K, Shimizu K. Hypogenesis of bilateral internal carotid artery. Shinkei. 1957; 9:37–43.3. Cho HJ, Jung YH, Kim YD, Nam HS, Kim DS, Heo JH. The different infarct patterns between adulthood-onset and childhood-onset moyamoya disease. J Neurol Neurosurg Psychiatr. 2011; 82:38–40. PMID: 20587492.
Article4. Ikezaki K, Matsushima T, Kuwabara Y, Suzuki SO, Nomura T, Fukui M. Cerebral circulation and oxygen metabolism in childhood moyamoya disease: a perioperative positron emission tomography study. J Neurosurg. 1994; 81:843–850. PMID: 7965114.
Article5. Abete P, Della-Morte D, Gargiulo G, Basile C, Langellotto A, Galizia G, et al. Cognitive impairment and cardiovascular diseases in the elderly: a heart-brain continuum hypothesis. Ageing Res Rev. 2014; 18:41–52. PMID: 25107566.
Article6. Hynes SM, Fish J, Manly T. Intensive working memory training: a single case experimental design in a patient following hypoxic brain damage. Brain Inj. 2014; 28:1766–1775. PMID: 25207877.
Article7. Kim JM, Lee SH, Roh JK. Changing ischaemic lesion patterns in adult moyamoya disease. J Neurol Neurosurg Psychiatr. 2009; 80:36–40. PMID: 18450791.
Article8. Kuroda S, Houkin K. Moyamoya disease: current concepts and future perspectives. Lancet Neurol. 2008; 7:1056–1066. PMID: 18940695.
Article9. Williams TS, Westmacott R, Dlamini N, Granite L, Dirks P, Askalan R, et al. Intellectual ability and executive function in pediatric moyamoya vasculopathy. Dev Med Child Neurol. 2012; 54:30–37. PMID: 22117564.
Article10. Festa JR, Schwarz LR, Pliskin N, Cullum CM, Lacritz L, Charbel FT, et al. Neurocognitive dysfunction in adult moyamoya disease. J Neurol. 2010; 257:806–815. PMID: 20033200.
Article11. Calviere L, Catalaa I, Marlats F, Januel AC, Lagarrigue J, Larrue V. Improvement in cognitive function and cerebral perfusion after bur hole surgery in an adult with moyamoya disease: case report. J Neurosurg. 2011; 115:347–349. PMID: 21529135.12. Jung HY, Park BK, Shin HS, Kang YK, Pyun SB, Paik NJ, et al. Development of the Korean Version of Modified Barthel Index (K-MBI): multi-center study for subjects with stroke. J Korean Acad Rehabil Med. 2007; 31:283–297.13. Kim YH, Shin SH, Park SH, Ko MH. Cognitive assessment for patient with brain injury by computerized neuropsychological test. J Korean Acad Rehabil Med. 2001; 25:209–216.14. Weinberg DG, Arnaout OM, Rahme RJ, Aoun SG, Batjer HH, Bendok BR. Moyamoya disease: a review of histopathology, biochemistry, and genetics. Neurosurg Focus. 2011; 30:E20. PMID: 21631222.
Article15. Hayashi K, Horie N, Izumo T, Nagata I. A nationwide survey on unilateral moyamoya disease in Japan. Clin Neurol Neurosurg. 2014; 124:1–5. PMID: 24976021.
Article16. Milner B. Some cognitive effects of frontal-lobe lesions in man. Philos Trans R Soc Lond B Biol Sci. 1982; 298:211–226. PMID: 6125972.17. Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim YH, Meyer JR, et al. A large-scale distributed network for covert spatial attention: further anatomical delineation based on stringent behavioural and cognitive controls. Brain. 1999; 122(Pt 6):1093–1106. PMID: 10356062.18. Black S, Gao F, Bilbao J. Understanding white matter disease: imaging-pathological correlations in vascular cognitive impairment. Stroke. 2009; 40(3 Suppl):S48–S52. PMID: 19064767.19. Kurumatani T, Kudo T, Ikura Y, Takeda M. White matter changes in the gerbil brain under chronic cerebral hypoperfusion. Stroke. 1998; 29:1058–1062. PMID: 9596257.
Article20. Riddle A, Dean J, Buser JR, Gong X, Maire J, Chen K, et al. Histopathological correlates of magnetic resonance imaging-defined chronic perinatal white matter injury. Ann Neurol. 2011; 70:493–507. PMID: 21796666.
Article21. Shibata M, Ohtani R, Ihara M, Tomimoto H. White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke. 2004; 35:2598–2603. PMID: 15472111.
Article22. Volkan-Salanci B, Lay Ergun E, Genc Sel C, Yalnizoglu D, Turanli G. The role of brain perfusion SPECT in moyamoya disease. Rev Esp Med Nucl Imagen Mol. 2012; 31:216–218. PMID: 22980130.
Article23. Kim SK, Oh JK, Lee EJ. Neurobehavioral cognitive status examination in stroke patients. J Korean Acad Rehabil Med. 1997; 21:259–263.24. Adamski N, Adler M, Opwis K, Penner IK. A pilot study on the benefit of cognitive rehabilitation in Parkinson's disease. Ther Adv Neurol Disord. 2016; 9:153–164. PMID: 27134671.
Article25. Feng H, Li G, Xu C, Ju C, Qiu X. Training rehabilitation as an effective treatment for patients with vascular cognitive impairment with no dementia. Rehabil Nurs. 2016.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Preliminary Study of Neurocognitive Dysfunction in Adult Moyamoya Disease and Improvement after Superficial Temporal Artery-Middle Cerebral Artery Bypass
- Neuroimaging Diagnosis and Treatment of Moyamoya Disease
- Clinical Study on the Obstructive Cerebrovascular Disease
- Moyamoya-like Disease Associated with Intracranial Aneurysm
- Changes of flow pattern after extracranial intracranial arterial bypass in patients with artherosclerotic cerebral ischemia and moyamoya disease