Lab Med Online.
2017 Jul;7(3):111-119. 10.3343/lmo.2017.7.3.111.
Performance Evaluation of the JEOL BioMajesty JCA-BM6010/C Automated Clinical Chemistry Analyzer
- Affiliations
-
- 1Department of Laboratory Medicine, Konkuk University School of Medicine, Seoul, Korea. ymyun@kuh.ac.kr
- 2Department of Laboratory Medicine, VHS Medical Center, Seoul, Korea.
- KMID: 2383089
- DOI: http://doi.org/10.3343/lmo.2017.7.3.111
Abstract
- BACKGROUND
JEOL BioMajesty JCA-BM6010/C (JCA-BM6010/C, JEOL Ltd., Japan) is a recently developed ultra-compact automated clinical chemistry analyzer with a throughput of 1,200 tests per hour. Here, we present the first performance evaluation of JCA-BM6010/C.
METHODS
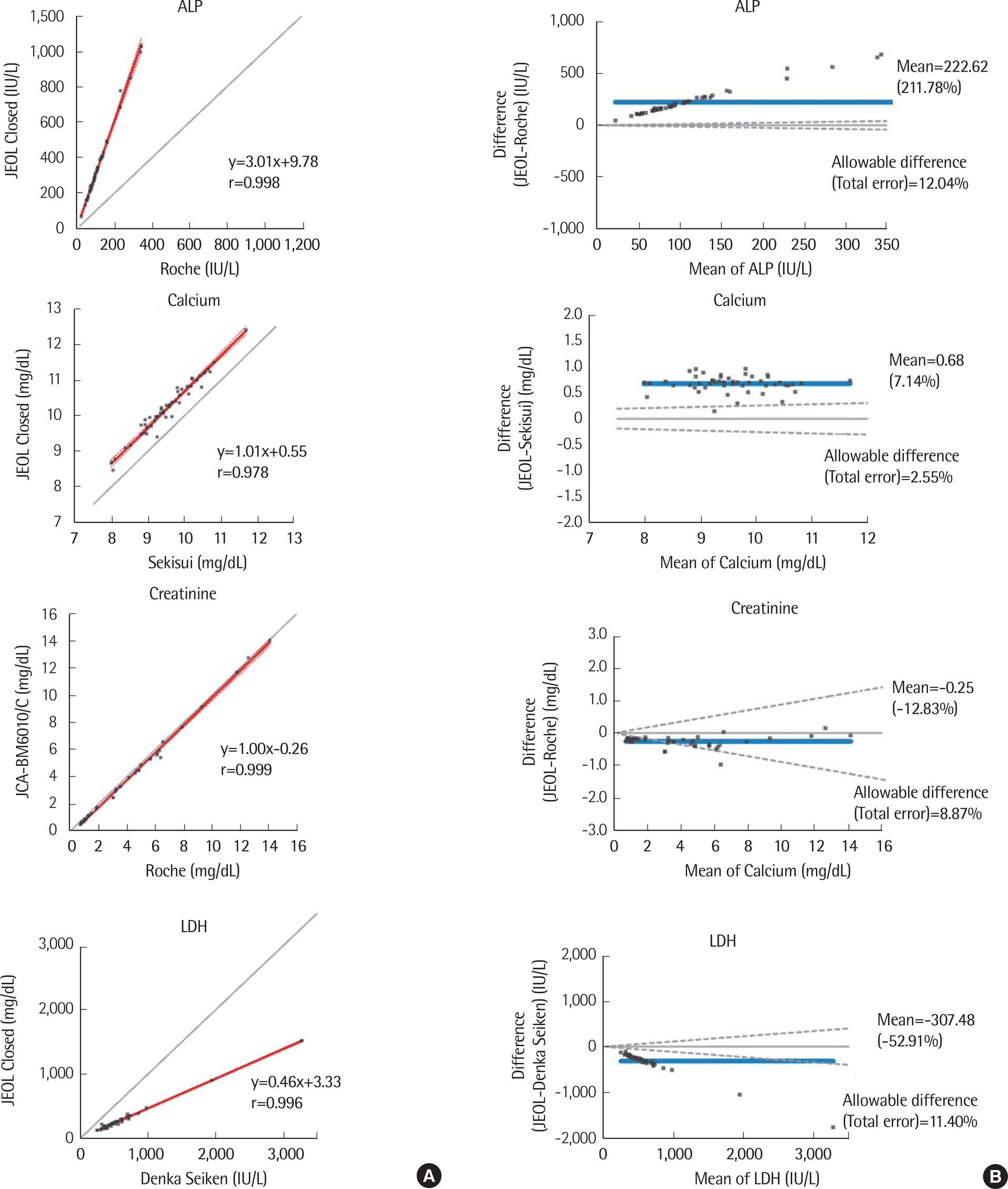
We evaluated the precision, linearity, correlation, accuracy, and carryover of 11 analytes (ALP, ALT, AST, calcium, creatinine, GGT, glucose, LDH, total bilirubin, total protein, and uric acid) using the JEOL closed reagent (JEOL Ltd.) according to the guidelines of the Clinical Laboratory Standards Institute. Linearity was further evaluated for ALT, AST, and GGT using open reagents by Sekisui (Japan). The performance of JCA-BM6010/C was compared to that of the Roche-Hitachi Cobas 8000 c702 chemistry autoanalyzer (Cobas 8000, Roche Diagnostics, Switzerland). Its performance using open reagents from Denka Seiken (Japan), Roche, and Sekisui was also evaluated.
RESULTS
The total coefficients of variation (CV) for all analytes were 1.0-2.7%. Linearity was observed for all analytes over the entire tested analytical range (R²â‰¥0.99). The results of JCA-BM6010/C strongly correlated (r≥0.988) with those of Cobas 8000 for all evaluated analytes except LDH (r=0.963), as well as for all open reagents. Recovery rates for creatinine, glucose, calcium, and uric acid were 96.6-101.5% and 98.7-109.3% with the JEOL exclusive and open reagents, respectively. Sample carryover was less than 0.34%.
CONCLUSIONS
JCA-BM6010/C showed acceptable performance in the precision, linearity, correlation, accuracy, and sample carryover analyses and in the method comparison. Therefore, it could be a useful routine laboratory medical analyzer.
MeSH Terms
Figure
Reference
-
1. Alpert NL. Automated instruments for clinical chemistry: review and preview. Clin Chem. 1969; 15:1198–209.
Article2. Lee DH, Yoon KJ. Evaluation of the Dimension Vista 1500 chemical autoanalyzer. J Lab Med Qual Assur. 2012; 34:77–86.3. Clinical and Laboratory Standards Institute. Evaluation of precision performance of quantitative measurement methods; approved guideline. 2nd ed.EP5-A2. Wayne, PA: Clinical and Laboratory Standards Institute;2004.4. Ricós C, Alvarez V, Cava F, García-Lario JV, Hernández A, Jiménez CV, et al. Desirable specifcation for total error, imprecision, and bias, derived from intra- and inter-individual biologic variation. http://www.westgard.com/biodatabase1.htm. (updated for 2014).5. Kim SY, Jeong TD, Lee W, Chun S, Min WK. Performance evaluation of the Roche-Hitachi cobas 8000 c702 chemistry autoanalyzer. Lab Med Online. 2014; 4:132–9.
Article6. Kim SK, Jeong TD, Lee W, Chun S, Min WK. Performance evaluation of Beckman Coulter AU5822 automated clinical chemistry analyzer. Lab Med Online. 2014; 4:77–84.
Article7. Clinical and Laboratory Standards Institute. Evaluation of the linearity of quantitative measurement procedures: A statistical approach; approved guideline. EP6-A. Wayne, PA: Clinical and Laboratory Standards Institute. 2003.8. Medicare, Medicaid and CLIA programs; regulations implementing the Clinical Laboratory Improvement Amendments of 1988 (CLIA)—HCFA. Final rule with comment period. Fed Regist. 1992; 57:7002–186.9. Clinical and Laboratory Standards Institute. Method comparison and bias estimation using patient samples; approved guideline. 2nd ed.EP9-A2. Wayne, PA: Clinical and Laboratory Standards Institute;2002.10. Clinical and Laboratory Standards Institute. Assessment of the diagnostic accuracy of laboratory tests using receiver operating characteristic curves; approved guideline. 2nd ed.EP15-A2. Wayne, PA: Clinical and Laboratory Standards Institute;2011.11. Clinical and Laboratory Standards Institute. Preliminary evaluation of quantitative clinical laboratory methods; approved guideline. 2nd ed.EP10-A2. Wayne, PA: Clinical and Laboratory Standards Institute;2002.12. Broughton PM. Carry-over in automatic analysers. J Automat Chem. 1984; 6:94–5.
Article13. Biswas SS, Bindra M, Jain V, Gokhale P. Evaluation of imprecision, bias and total error of clinical chemistry analysers. Ind J Clin Biochem. 2015; 30:104–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analytical Evaluation of the DiaSys Albumin in Urine/CSF FS Kit for Urine Albumin Measurement Using a JEOL BioMajesty JCA-BM6010/C Analyzer
- Performance Evaluation of Beckman Coulter AU5822 Automated Clinical Chemistry Analyzer
- Performance Evaluation of the CLINITEK Novus Automated Urine Chemistry Analyzer
- Evaluation of the Analytical Performance of Atellica CH 930 Automated Chemistry Analyzer
- Evaluation of Yeong Dong Reagent for the Automated Chemistry Test