Lab Med Online.
2016 Apr;6(2):64-69. 10.3343/lmo.2016.6.2.64.
Analytical Evaluation of the DiaSys Albumin in Urine/CSF FS Kit for Urine Albumin Measurement Using a JEOL BioMajesty JCA-BM6010/C Analyzer
- Affiliations
-
- 1Department of Laboratory Medicine, Konkuk University School of Medicine, Seoul, Korea. ymyun@kuh.ac.kr
- KMID: 2160086
- DOI: http://doi.org/10.3343/lmo.2016.6.2.64
Abstract
- BACKGROUND
High albuminuria is defined as albumin excretion of >30 mg/24 hr or an albumin-to-creatinine ratio of 30 mg/g in a random urine sample. We assessed the analytical performance of the Albumin in Urine/CSF FS kit (DiaSys Inc., UK) using a BioMajesty JCA-6010/C analyzer (JEOL Inc., Japan).
METHODS
Urine albumin concentrations were measured by the Albumin in Urine/CSF FS kit using a BioMajesty JCA-BM6010/C analyzer. Imprecision, linearity, and carry-over were measured according to the Clinical Laboratory and Standards Institute documents EP10 and EP9. The assay was compared with the ALB-T TQ Gen.2 (Roche, Germany) assay on a Cobas8000 C702 (Roche, Germany), the Tina-Quant Albumin (Roche, Switzerland) assay on a Hitachi7600-210 (Hitachi, Japan), and an Abbott urine albumin assay (Abbott Laboratories, USA) on a TBA 200FR (Toshiba, Japan) using 50 random urine samples.
RESULTS
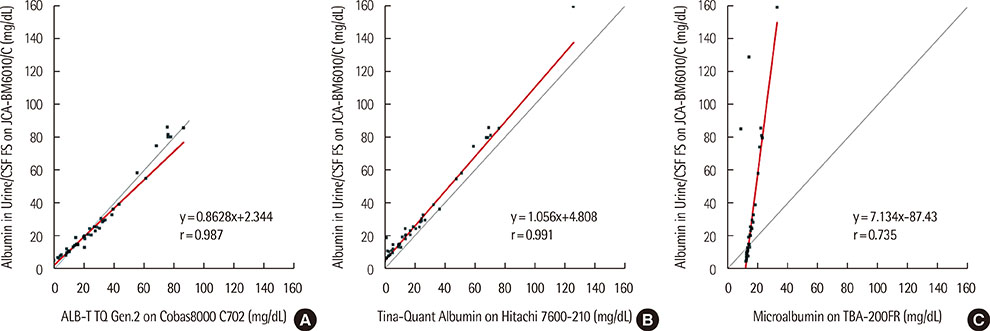
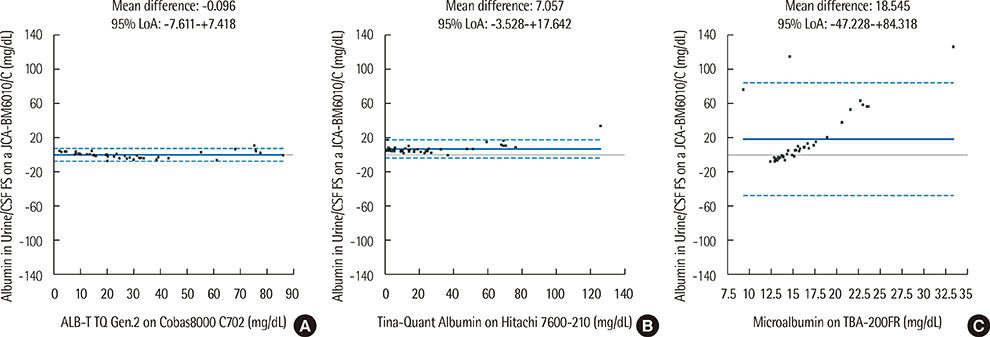
Within-run and total imprecision were 0.551-1.023% and 0.551-1.214%, respectively. Linearity ranged from 6.31 to 30.60 mg/dL, and functional sensitivity was 0.5 mg/dL. Results from the Albumin in Urine/CSF FS kit showed good correlation with the ALB-T TQ Gen.2 (r=0.987) and the Tina-Quant Albumin assays (r=0.991). However, the four assays categorized 18 of 50 urine samples into different albuminuria groups.
CONCLUSIONS
Albumin in Urine/CSF FS testing on a BioMajesty JCA-BM6010/C analyzer showed good linearity, functional sensitivity, precision, and correlation with the ALB-T TQ Gen.2 and Tina-Quant Albumin assays. However, because some samples were categorized into different albuminuria groups by the different assays, further studies on the standardization of albuminuria assays are needed.
MeSH Terms
Figure
Reference
-
1. Levey AS, Cattran D, Friedman A, Miller WG, Sedor J, Tuttle K, et al. Proteinuria as a surrogate outcome in CKD: report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2009; 54:205–226.
Article2. The Korean Society for Laboratory Medicine. Laboratory Medicine. 5th ed. Seoul: Beommun Education;2014. p. 15p. 359p. 500–501.3. Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002; 106:1777–1782.
Article4. Kidney Disease Outcomes Quality Initiative. National kidney practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003; 139:137–147.5. Clinical and Laboratory Standards Institute. Preliminary evaluation of quantitative clinical measurement procedures; approved guideline. CLSI document EP10-A3. 3rd ed. Wayne, PA: Clinical and Laboratory Standards Institute;2014.6. Clinical and Laboratory Standards Institute. Method comparison and bias estimation using patient samples; approved guideline. CLSI document EP9-A2. 2nd ed. Wayne, PA: Clinical and Laboratory Standards Institute;2002.7. DiaSys Diagnostics Systems GmbH. Reagent information of Albumin in Urine/CSF FS. 2011. Feb.8. Cho JH, Lee CM, Park CM, Moon HW, Hur M, Yun YM, et al. Evaluation of the performance of Lumipulse G1200 for tumor marker assays. Lab Med Online. 2012; 2:131–138.
Article9. Ricos C, Alvarez V, Cava F, Garcia-Lario JV, Hernandez A, Jimenez CV, et al. Desirable specifications for total error, imprecision, and bias, derived from intra- and inter-individual biologic variation. updated in 2014. https://www.westgard.com/biodatabase1.htm#im.10. Fraser CG, Harris EK. Generation and application of data on biological variation in clinical chemistry. Crit Rev Clin Lab Sci. 1989; 27:409–437.
Article11. Ricós C, Alvarez V, Cava F, García-Lario JV, Hernández A, Jiménez CV, et al. Current databases on biological variation: pros, cons and progress. Scand J Clin Lab Invest. 1999; 59:491–500.
Article12. National Kidney Foundation. K/DOQI Clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002; 39:S1–S266.13. Warram JH, Gearin G, Laffel L, Krolewski AS. Effect of duration of type I diabetes on the prevalence of stages of diabetic nephropathy defined by urinary albumin/creatinine ratio. J Am Soc Nephrol. 1996; 7:930–937.
Article14. Jensen JS, Feldt-Rasmussen B, Strandgaard S, Schroll M, Borch-Johnsen K. Arterial hypertension, microalbuminuria, and risk of ischemic heart disease. Hypertension. 2000; 35:898–903.
Article15. Architect. Reagent information of Microalbumin. 2004. Dec.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Performance Evaluation of the JEOL BioMajesty JCA-BM6010/C Automated Clinical Chemistry Analyzer
- Relationship and Clinical Significance of Urine Protein between Serum Albumin Level in Pregnacy Induced Hypertension
- Estimation of Microalbuminuria by Urinary Albumin to Creatinine Concentration Ratio
- Performance Evaluation of the Afinion AS100 Analyzer for Its Use in the Albumin Creatinine Ratio Test
- Performance Evaluation of Urine Dipstick Analyzer CYBOW R-600S