Lab Med Online.
2019 Jul;9(3):133-145. 10.3343/lmo.2019.9.3.133.
Evaluation of the Analytical Performance of Atellica CH 930 Automated Chemistry Analyzer
- Affiliations
-
- 1Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea. comforter6@yuhs.ac
- 2Department of Laboratory Medicine, Wonju Severance Christian Hospital, Yonsei University Wonju College of Medicine, Wonju, Korea.
- KMID: 2450838
- DOI: http://doi.org/10.3343/lmo.2019.9.3.133
Abstract
- BACKGROUND
Recently, a new automated chemistry analyzer, Atellica CH930 (Siemens, Germany), was introduced. It automatically measures internal quality control (QC) materials according to a pre-determined schedule. For this purpose, the instrument has space for storage of QC materials. We evaluated the analytical performance of chemistry items by using the Atellica system.
METHODS
The precision of 29 items was evaluated with three levels of QC materials with two storage methods. We stored the QC materials in the dedicated storage space in the instrument during the precision evaluation period. In addition, we aliquoted and stored the materials in the refrigerator, and then loaded the material in a timely manner. Linearity, carry-over, and agreement with current methods were also evaluated.
RESULTS
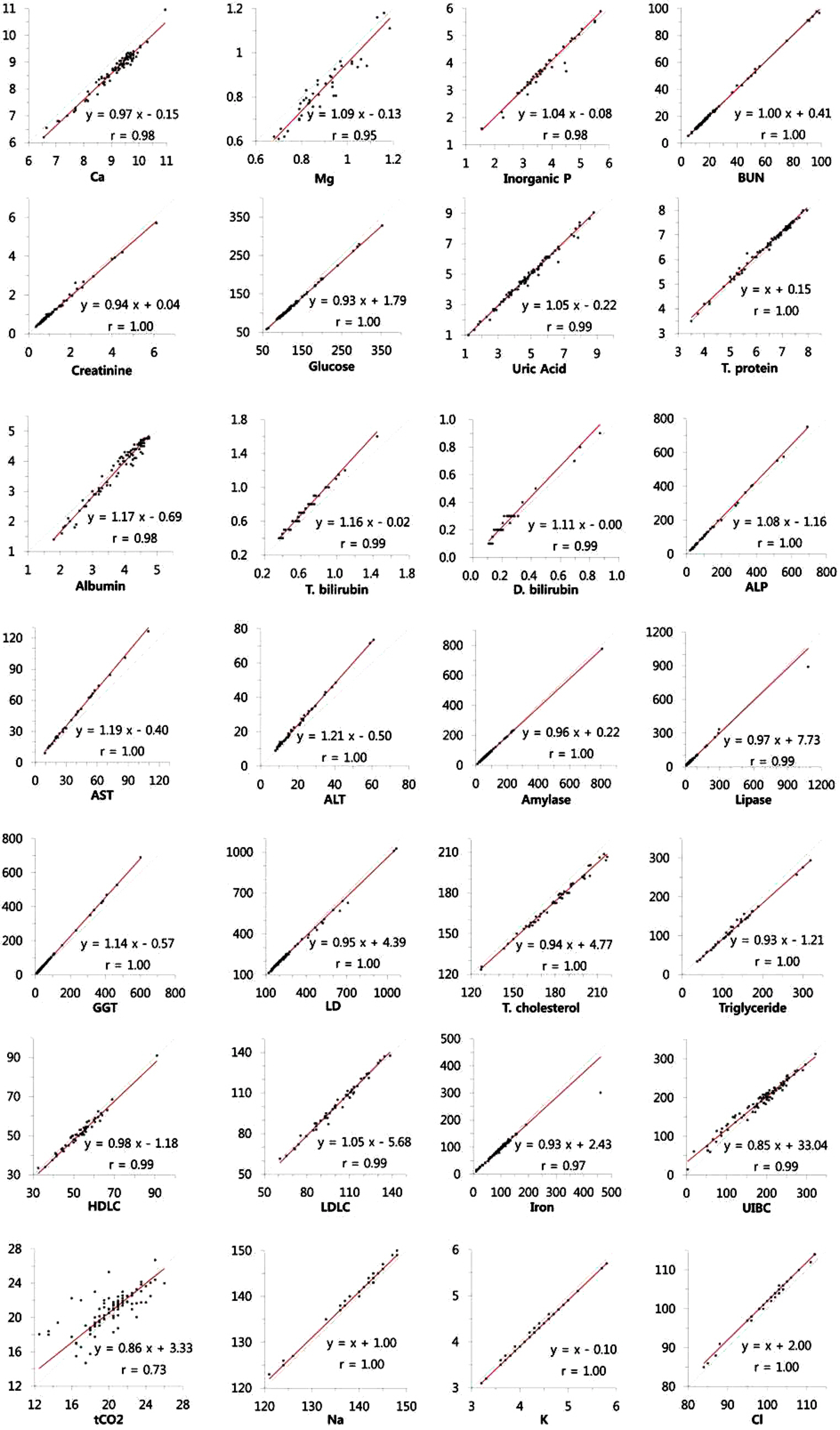
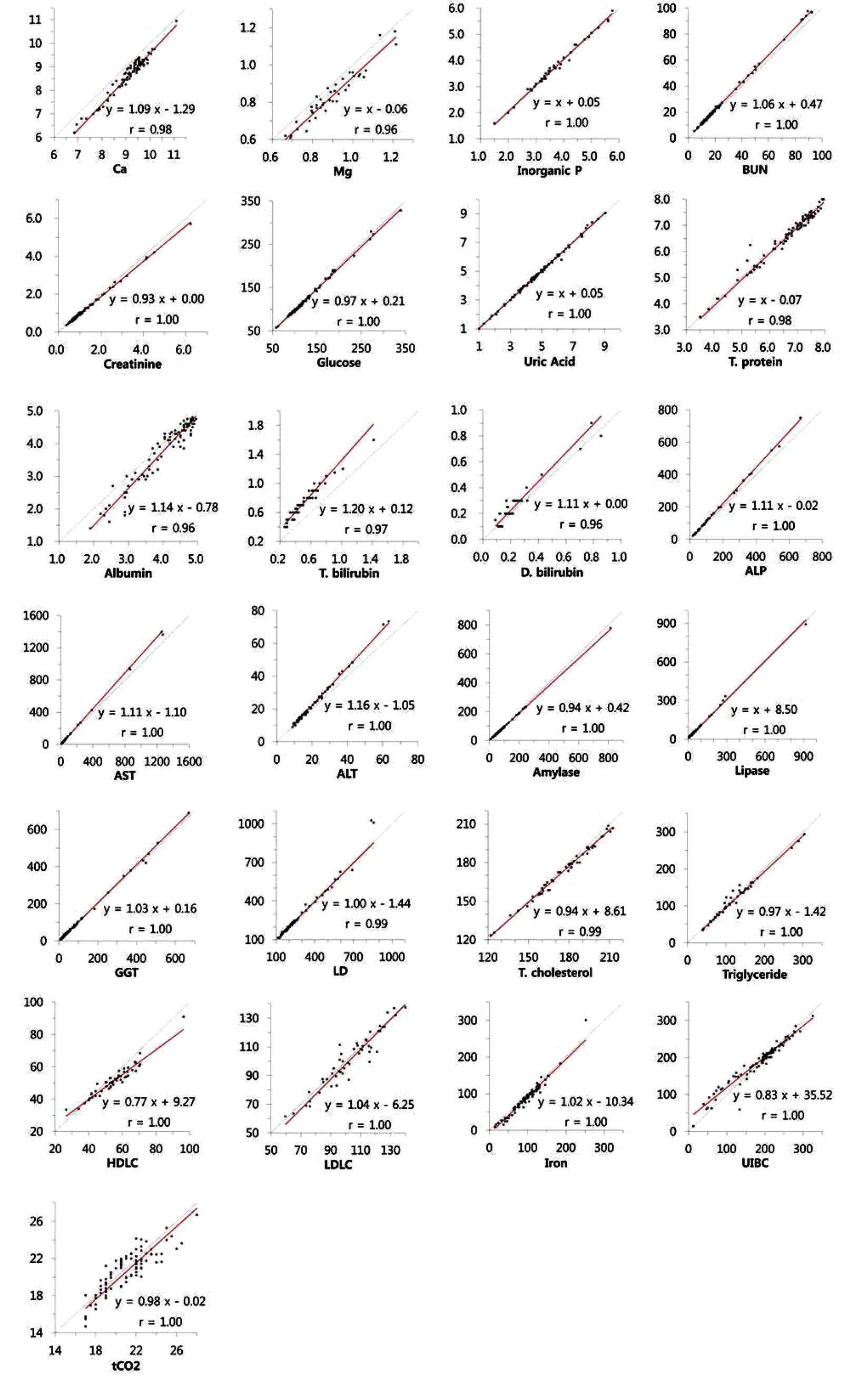
The within-laboratory coefficient of variation (CV) of most items, except for total CO2 (tCO2), was within 5.0% in both QC storage methods without significant differences in CV between storage methods. The CV of tCO2 was 5.2%, 5.8%, and 5.1% at three different levels when the QC materials were stored in a dedicated space in the instrument. The linearity was acceptable, showing <5% nonlinearity. Although good agreement was observed for most items, some items, such as calcium, total bilirubin, aspartate transaminase, and chloride, showed unequivalent results.
CONCLUSIONS
Atellica CH930 showed acceptable precision, linearity, and agreement in routine chemistry items. The automatic QC function using the storage device has no problem with stability or precision. It can reduce the manual process, allowing technicians to focus on reviewing the QC results and reporting reliable results.
MeSH Terms
Figure
Reference
-
References
1. Brombacher PJ, Marell GJ, Westerhuis LW. Laboratory work fow analysis and introduction of a multifunctional analyser. Eur J Clin Chem Clin Biochem. 1996; 34:287–92.2. Armbruster DA, Overcash DR, Reyes J. Clinical chemistry laboratory automation in the 21st century – Amat Victoria curam (Victory loves careful preparation). Clin Biochem Rev. 2014; 35:143–53.3. Dolci A, Giavarina D, Pasqualetti S, Szőke D, Panteghini M. Total laboratory automation: Do stat tests still matter? Clin Biochem. 2017; 50:605–11.
Article4. Lawson NS, Haven GT, Williams GW. Analyte stability in clinical chemistry quality control materials. Crit Rev Clin Lab Sci. 1982; 17:1–50.
Article5. Clinical and Laboratory Standards Institute. Evaluation of precision of quantitative measurement procedures; approved guideline—third edition. CLSI document EP05-A3. Wayne, PA: Clinical and Laboratory Standards Institute;2014.6. Clinical and Laboratory Standards Institute. Evaluation of the of the linearity of quantitative measurement procedures: a statistical approach; approved guideline. CLSI document EP06-A. Wayne, PA: Clinical and Laboratory Standards Institute;2003.7. Clinical and Laboratory Standards Institute. Method comparison and bias estimation using patient samples; approved guideline—second edition (interim revision). CLSI document EP09-A2-IR. Wayne, PA: Clinical and Laboratory Standards Institute;2010.8. Statland BE, ed. Clinical decision levels for laboratory tests. 2nd ed.Oradell, NJ: Medical Economics Books;1987.9. Burtis CA, Ashwood ER, et al. eds. Tietz textbook of clinical chemistry and molecular diagnostics. 4th ed.St. Louis: Elsevier-Saunders;2006. p. 363–4.10. Grundy SM, Becker D, Clark LT, Cooper RS, Denke MA, Howard J, et al. Detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Circulation. 2002; 106:3143–421.11. Ricos C, Alvarez V, Cava F, Garcia-Lario JV, Hernandez A, Jimenez CV, et al. Desirable specifcations for total error, imprecision, and bias, derived from intra- and interindividual biologic variation. https://www.westgard.com/biodatabase1.htm. (Updated in 2014).12. Westgard QC. CLIA requirements for analytical quality. https://www.westgard.com/clia.htm. (Updated on Feb, 1992).13. Burtis CA, Ashwood ER, et al. eds. Tietz textbook of clinical chemistry and molecular diagnostics. 5th ed.Philadelphia: Elsevier-Saunders;2014.14. Westgard QC. Royal College of Pathologists of Australasia analytical quality requirements. https://www.westgard.com/rcpa-biochemistry. htm#serumchemistry (Updated in 2013).15. Greg Miller W, Myers GL, Lou Gantzer M, Kahn SE, Schönbrunner ER, Thienpont LM, et al. Roadmap for harmonization of clinical laboratory measurement procedures. Clin Chem. 2011; 57:1108–17.
Article16. Klauke R, Kytzia HJ, Weber F, Grote-Koska D, Brand K, Schumann G. Reference measurement procedure for total bilirubin in serum re-evaluated and measurement uncertainty determined. Clin Chim Acta. 2018; 481:115–20.
Article17. Lo SF, Kytzia HJ, Schumann G, Swartzentruber M, Vader HL, Weber F, et al. Interlaboratory comparison of the Doumas bilirubin reference method. Clin Biochem. 2009; 42:1328–30.
Article18. Lo S, Jendrzejczak B, Doumas BT. Bovine serum–based bilirubin calibrators are inappropriate for some diazo methods. Clin Chem. 2010; 56:869–72.
Article19. Infusino I, Frusciante E, Braga F, Panteghini M. Progress and impact of enzyme measurement standardization. Clin Chem Lab Med. 2017; 55:334–40.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Performance Evaluation of Beckman Coulter AU5822 Automated Clinical Chemistry Analyzer
- A Comparative Study of Biological and Analytical Variability of Automated Clinical Chemistry Tests
- Performance Evaluation of the JEOL BioMajesty JCA-BM6010/C Automated Clinical Chemistry Analyzer
- Performance Evaluation of the CLINITEK Novus Automated Urine Chemistry Analyzer
- Evaluation of Analytical Performance of an Automated Glycated Hemoglobin Analyzer, HLC-723 G11