Ann Dermatol.
2016 Oct;28(5):600-606. 10.5021/ad.2016.28.5.600.
Acute Stress-Induced Changes in Follicular Dermal Papilla Cells and Mobilization of Mast Cells: Implications for Hair Growth
- Affiliations
-
- 1Department of Dermatology, Dongguk University Ilsan Hospital, Goyang, Korea.
- 2Department of Dermatology, Seoul National University College of Medicine, Seoul, Korea. oskwon@snu.ac.kr
- 3Laboratory of Cutaneous Aging and Hair Research, Biomedical Research Institute, Seoul National University Hospital, Seoul, Korea.
- 4Institute of Human-Environment Interface Biology, Medical Research Center, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2382885
- DOI: http://doi.org/10.5021/ad.2016.28.5.600
Abstract
- BACKGROUND
Stress is a known cause of hair loss in many species.
OBJECTIVE
In this study, we investigated the role of acute stress on hair growth using a rat model.
METHODS
Rats were immobilized for 24 hours and blood samples, and skin biopsies were taken. The effect of stress-serum on the in vitro proliferation of rat and human dermal papilla cells (hDPCs), as well as serum cortisol and corticotropin-releasing hormone levels, were measured. Mast cell staining was performed on the biopsied tissue. In addition, Western blot and quantitative real time polymerase chain reaction were used to assess mast cell tryptase and cytokine expression, respectively in rat skin biopsies.
RESULTS
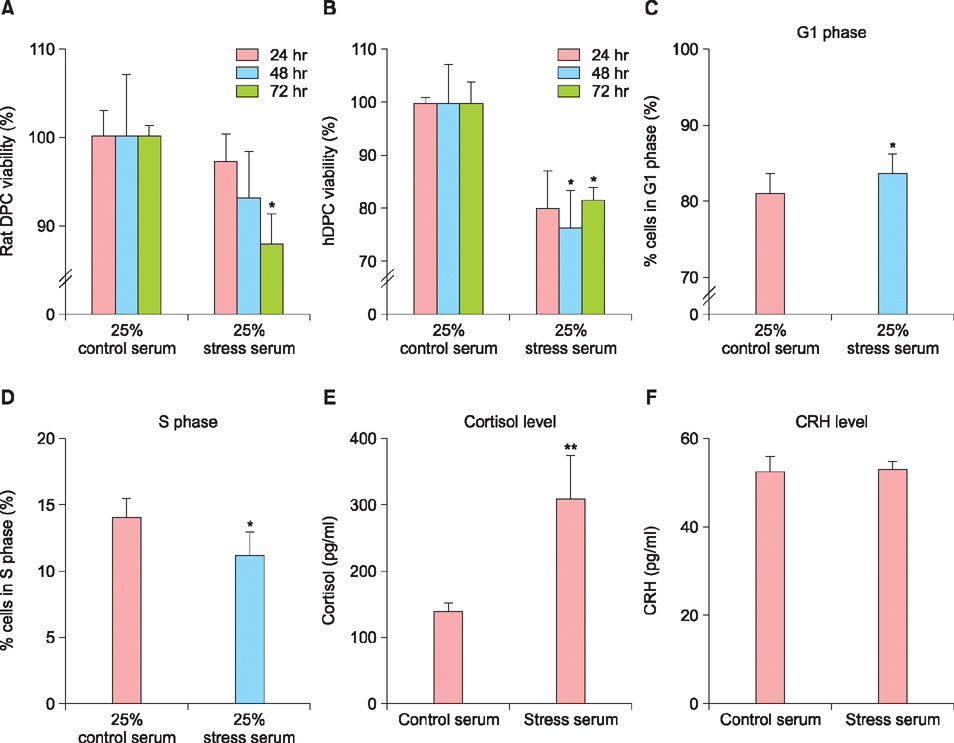
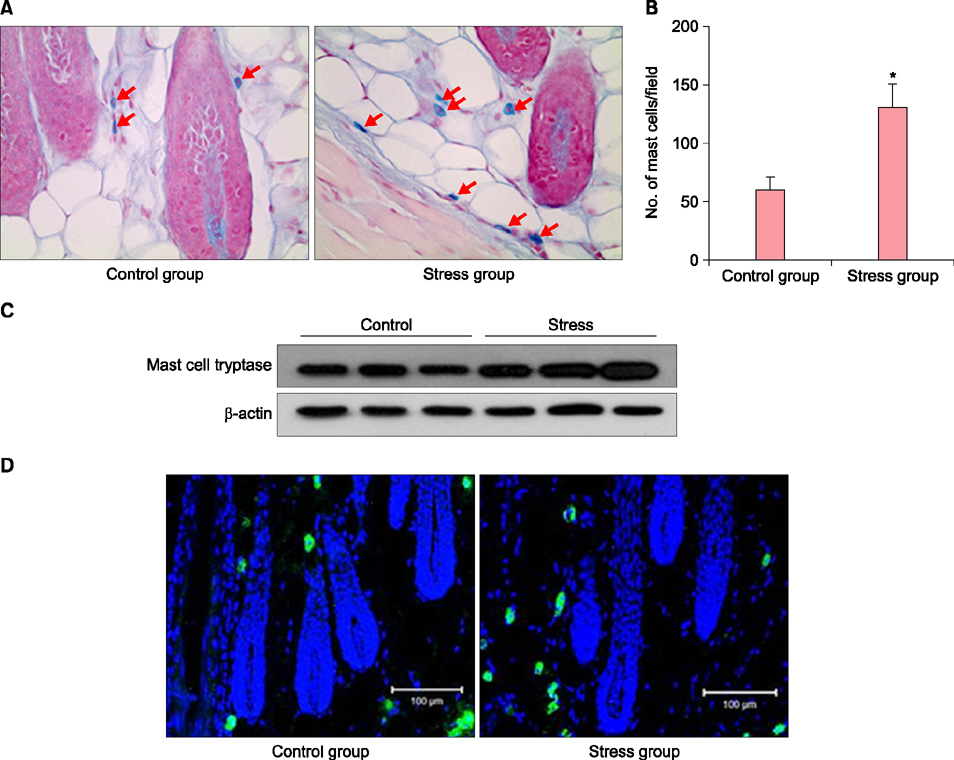
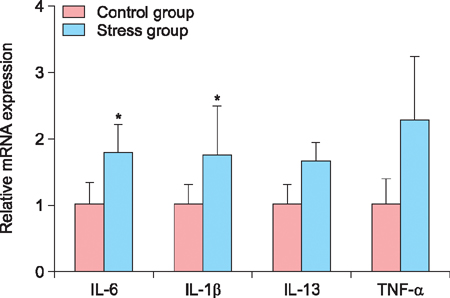
Stress-serum treatment reduced significantly the number of viable hDPCs and arrested the cell cycle in the G1 phase, compared to serum from unrestrained rats (p<0.05, respectively). Moreover, restrained rats had significantly higher levels of cortisol in serum than unrestrained rats (p<0.01). Acute stress serum increased mast cell numbers and mast cell tryptase expression, as well as inducing interleukin (IL)-6 and IL-1β up-regulation.
CONCLUSION
These results suggest that acute stress also has an inhibitory effect on hair growth via cortisol release in addition to substance P-mast cell pathway.
Keyword
MeSH Terms
Figure
Reference
-
1. Hadshiew IM, Foitzik K, Arck PC, Paus R. Burden of hair loss: stress and the underestimated psychosocial impact of telogen effluvium and androgenetic alopecia. J Invest Dermatol. 2004; 123:455–457.
Article2. Manolache L, Petrescu-Seceleanu D, Benea V. Alopecia areata and relationship with stressful events in children. J Eur Acad Dermatol Venereol. 2009; 23:107–109.
Article3. Chu SY, Chen YJ, Tseng WC, Lin MW, Chen TJ, Hwang CY, et al. Psychiatric comorbidities in patients with alopecia areata in Taiwan: a case-control study. Br J Dermatol. 2012; 166:525–531.
Article4. Manolache L, Benea V. Stress in patients with alopecia areata and vitiligo. J Eur Acad Dermatol Venereol. 2007; 21:921–928.
Article5. Atefi N, Soltani-Arabshahi R, Afkham-Ebrahimi A. Stressful life events and diffuse unexplained hair loss in women: a case-control study. Dermatology. 2006; 213:44–45.
Article6. Arck PC, Handjiski B, Hagen E, Joachim R, Klapp BF, Paus R. Indications for a 'brain-hair follicle axis (BHA)': inhibition of keratinocyte proliferation and up-regulation of keratinocyte apoptosis in telogen hair follicles by stress and substance P. FASEB J. 2001; 15:2536–2538.
Article7. Arck PC, Slominski A, Theoharides TC, Peters EM, Paus R. Neuroimmunology of stress: skin takes center stage. J Invest Dermatol. 2006; 126:1697–1704.
Article8. Arck PC, Handjiski B, Kuhlmei A, Peters EM, Knackstedt M, Peter A, et al. Mast cell deficient and neurokinin-1 receptor knockout mice are protected from stress-induced hair growth inhibition. J Mol Med (Berl). 2005; 83:386–396.
Article9. Theoharides TC, Spanos C, Pang X, Alferes L, Ligris K, Letourneau R, et al. Stress-induced intracranial mast cell degranulation: a corticotropin-releasing hormone-mediated effect. Endocrinology. 1995; 136:5745–5750.
Article10. Esposito P, Chandler N, Kandere K, Basu S, Jacobson S, Connolly R, et al. Corticotropin-releasing hormone and brain mast cells regulate blood-brain-barrier permeability induced by acute stress. J Pharmacol Exp Ther. 2002; 303:1061–1066.
Article11. Chebotaev DV, Yemelyanov AY, Lavker RM, Budunova IV. Epithelial cells in the hair follicle bulge do not contribute to epidermal regeneration after glucocorticoid-induced cutaneous atrophy. J Invest Dermatol. 2007; 127:2749–2758.
Article12. Choi SJ, Cho AR, Jo SJ, Hwang ST, Kim KH, Kwon OS. Effects of glucocorticoid on human dermal papilla cells in vitro. J Steroid Biochem Mol Biol. 2013; 135:24–29.
Article13. Méndez-Cuesta LA, Márquez-Valadez B, Pérez-De La Cruz V, Escobar-Briones C, Galván-Arzate S, Alvarez-Ruiz Y, et al. Diazepam blocks striatal lipid peroxidation and improves stereotyped activity in a rat model of acute stress. Basic Clin Pharmacol Toxicol. 2011; 109:350–356.
Article14. Shin H, Cho AR, Kim DY, Munkhbayer S, Choi SJ, Jang S, et al. Enhancement of human hair Growth using ecklonia cava polyphenols. Ann Dermatol. 2016; 28:15–21.
Article15. Shin H, Yoo HG, Inui S, Itami S, Kim IG, Cho AR, et al. Induction of transforming growth factor-beta 1 by androgen is mediated by reactive oxygen species in hair follicle dermal papilla cells. BMB Rep. 2013; 46:460–464.
Article16. Yoon SY, Yoon JS, Jo SJ, Shin CY, Shin JY, Kim JI, et al. A role of placental growth factor in hair growth. J Dermatol Sci. 2014; 74:125–134.
Article17. Jo SJ, Choi SJ, Yoon SY, Lee JY, Park WS, Park PJ, et al. Valproic acid promotes human hair growth in in vitro culture model. J Dermatol Sci. 2013; 72:16–24.
Article18. Kwon OS, Pyo HK, Oh YJ, Han JH, Lee SR, Chung JH, et al. Promotive effect of minoxidil combined with all-trans retinoic acid (tretinoin) on human hair growth in vitro. J Korean Med Sci. 2007; 22:283–289.
Article19. Liu N, Wang LH, Guo LL, Wang GQ, Zhou XP, Jiang Y, et al. Chronic restraint stress inhibits hair growth via substance P mediated by reactive oxygen species in mice. PLoS One. 2013; 8:e61574.
Article20. Ghisleni G, Capiotti KM, Da Silva RS, Oses JP, Piato ÂL, Soares V, et al. The role of CRH in behavioral responses to acute restraint stress in zebrafish. Prog Neuropsychopharmacol Biol Psychiatry. 2012; 36:176–182.
Article21. Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997; 77:1033–1079.
Article22. Prignano F, Lotti T, Spallanzani A, Berti S, de Giorgi V, Moretti S. Sequential effects of photodynamic treatment of basal cell carcinoma. J Cutan Pathol. 2009; 36:409–416.
Article23. Yoshikawa H, Tasaka K. Suppression of mast cell activation by glucocorticoid. Arch Immunol Ther Exp (Warsz). 2000; 48:487–495.
Article24. Ito N, Sugawara K, Bodó E, Takigawa M, van Beek N, Ito T, et al. Corticotropin-releasing hormone stimulates the in situ generation of mast cells from precursors in the human hair follicle mesenchyme. J Invest Dermatol. 2010; 130:995–1004.
Article25. Peters EM, Arck PC, Paus R. Hair growth inhibition by psychoemotional stress: a mouse model for neural mechanisms in hair growth control. Exp Dermatol. 2006; 15:1–13.
Article26. Ilves T, Harvima IT. Decrease in chymase activity is associated with increase in IL-6 expression in mast cells in atopic dermatitis. Acta Derm Venereol. 2015; 95:411–416.
Article27. Kwack MH, Ahn JS, Kim MK, Kim JC, Sung YK. Dihydrotestosterone-inducible IL-6 inhibits elongation of human hair shafts by suppressing matrix cell proliferation and promotes regression of hair follicles in mice. J Invest Dermatol. 2012; 132:43–49.
Article28. Hoffmann R, Wenzel E, Huth A, van der Steen P, Schäufele M, Henninger HP, et al. Cytokine mRNA levels in alopecia areata before and after treatment with the contact allergen diphenylcyclopropenone. J Invest Dermatol. 1994; 103:530–533.
Article29. Swolin-Eide D, Ohlsson C. Effects of cortisol on the expression of interleukin-6 and interleukin-1 beta in human osteoblast-like cells. J Endocrinol. 1998; 156:107–114.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Alopecia Areata Serum on Proliferation of Cultured Dermal Papilla Cells
- Effects of Vaseular Endothelial Growth Factors on Hair Growth in Vitro
- Effect of Chrysanthemum zawadskii Extract on Dermal Papilla Cell Proliferation and Hair Growth
- Effects of Substance P on the Expression of Various Factors to control Hair Growth in Human Hair Follicle Culture
- Glycogen Phosphorylase Inhibitor Promotes Hair Growth via Protecting from Oxidative-Stress and Regulating Glycogen Breakdown in Human Hair follicles