J Pathol Transl Med.
2015 Nov;49(6):489-496. 10.4132/jptm.2015.09.09.
Chronic Placental Inflammation in Twin Pregnancies
- Affiliations
-
- 1Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. jsunkim@skku.edu
- 2Department of Pathology, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea.
- 3Department of Obstetrics and Gynecology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 4Department of Health Sciences and Technology, Sungkyunkwan University, SAIHST, Seoul, Korea.
- KMID: 2381408
- DOI: http://doi.org/10.4132/jptm.2015.09.09
Abstract
- BACKGROUND
Chronic placental inflammation, such as villitis of unknown etiology (VUE) and chronic chorioamnionitis (CCA), is considered a placental manifestation of maternal anti-fetal rejection. The aim of this study is to investigate its frequency in twin pregnancies compared to singleton pregnancies.
METHODS
Three hundred twin placentas and 1,270 singleton placentas were consecutively collected at a tertiary medical center in Seoul, Republic of Korea from 2009 to 2012. Hematoxylin and eosin sections of tissue samples (full-thickness placental disc and chorioamniotic membranes) were reviewed.
RESULTS
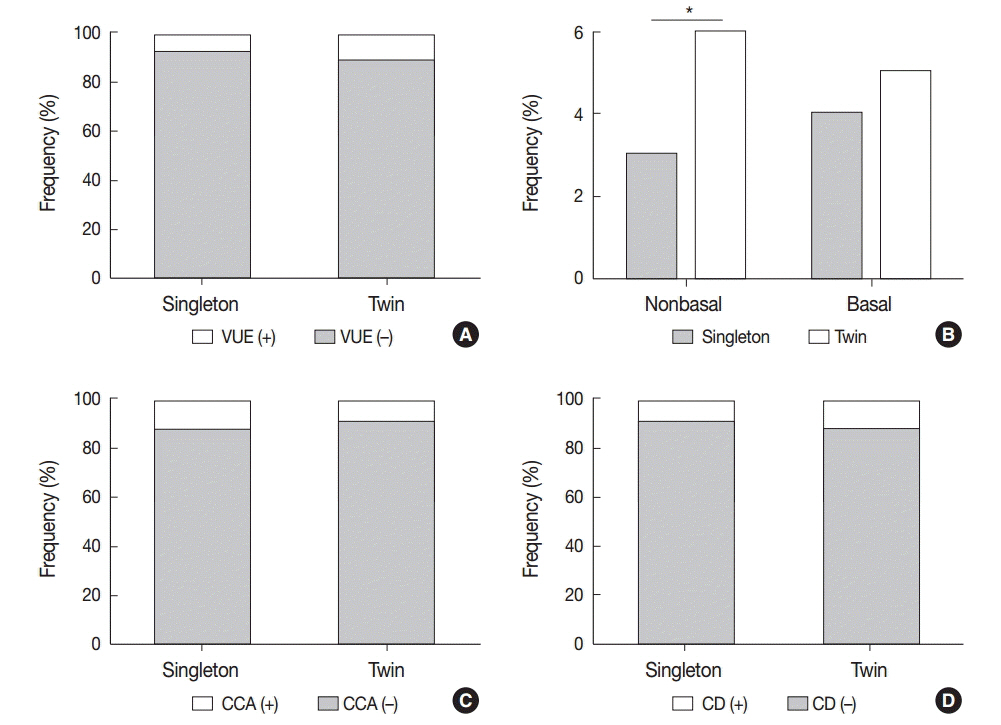
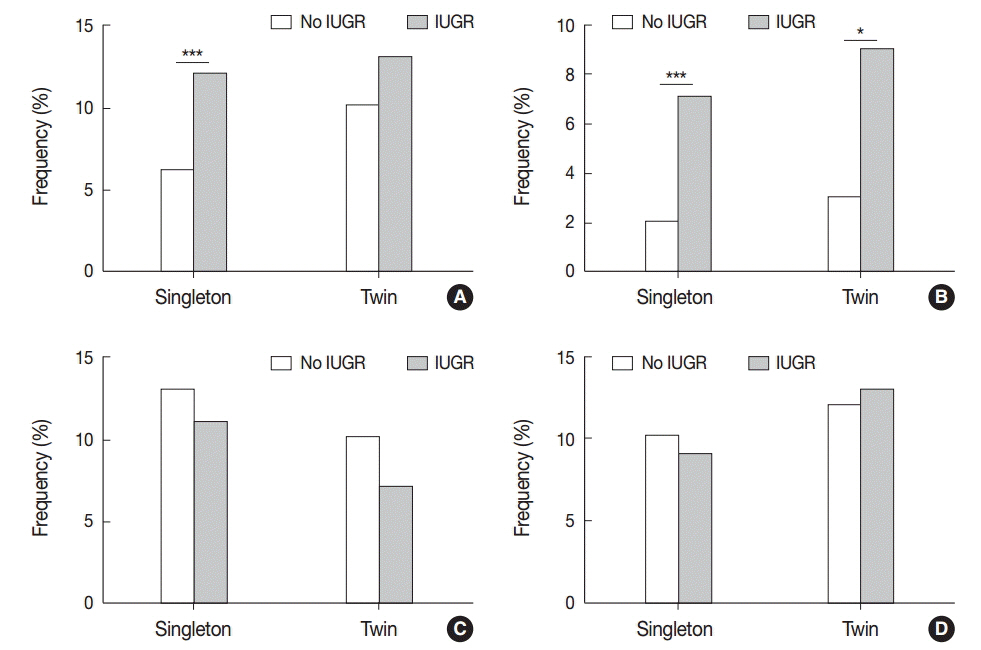
Non-basal VUE was more frequent in twin placentas than in singleton placentas (6.0% vs 3.2%, p < .05). In preterm birth, CCA was found less frequently in twin placentas than in singleton placentas (9.6% vs 14.8%, p < .05), reaching its peak at an earlier gestational age in twin placentas (29-32 weeks) than in singleton placentas (33-36 weeks). CCA was more frequent in twin pregnancies with babies of a different sex than with those with the same sex (13.8% vs 6.9%, p=.052). Separate dichorionic diamniotic twin placentas were affected by chronic deciduitis more frequently than singleton placentas (16.9% vs 9.7%, p<.05).
CONCLUSIONS
The higher frequency of non-basal VUE in twin placentas and of CCA in twin placentas with different fetal sex supports the hypothesis that the underlying pathophysiological mechanism is maternal anti-fetal rejection related to increased fetal antigens in twin pregnancies. The peak of CCA at an earlier gestational age in twin placentas than in singleton placentas suggests that CCA is influenced by placental maturation.
Keyword
MeSH Terms
Figure
Reference
-
1. Kim JS, Romero R, Kim MR, et al. Involvement of Hofbauer cells and maternal T cells in villitis of unknown aetiology. Histopathology. 2008; 52:457–64.
Article2. Boog G. Chronic villitis of unknown etiology. Eur J Obstet Gynecol Reprod Biol. 2008; 136:9–15.
Article3. Derricott H, Jones RL, Heazell AE. Investigating the association of villitis of unknown etiology with stillbirth and fetal growth restriction: a systematic review. Placenta. 2013; 34:856–62.4. Redline RW, Ariel I, Baergen RN, et al. Fetal vascular obstructive lesions: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2004; 7:443–52.
Article5. Gersell DJ, Phillips NJ, Beckerman K. Chronic chorioamnionitis: a clinicopathologic study of 17 cases. Int J Gynecol Pathol. 1991; 10:217–29.6. Jacques SM, Qureshi F. Chronic chorioamnionitis: a clinicopathologic and immunohistochemical study. Hum Pathol. 1998; 29:1457–61.
Article7. Kim CJ, Romero R, Kusanovic JP, et al. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod Pathol. 2010; 23:1000–11.
Article8. Lee J, Kim JS, Park JW, et al. Chronic chorioamnionitis is the most common placental lesion in late preterm birth. Placenta. 2013; 34:681–9.
Article9. Lee J, Romero R, Dong Z, et al. Unexplained fetal death has a biological signature of maternal anti-fetal rejection: chronic chorioamnionitis and alloimmune anti-human leucocyte antigen antibodies. Histopathology. 2011; 59:928–38.
Article10. Khong TY, Bendon RW, Qureshi F, et al. Chronic deciduitis in the placental basal plate: definition and interobserver reliability. Hum Pathol. 2000; 31:292–5.11. Bendon RW, Miller M. Routine pathological examination of placentae from abnormal pregnancies. Placenta. 1990; 11:369–70.12. Edmondson N, Bocking A, Machin G, Rizek R, Watson C, Keating S. The prevalence of chronic deciduitis in cases of preterm labor without clinical chorioamnionitis. Pediatr Dev Pathol. 2009; 12:16–21.
Article13. Yoo C, Jang DG, Jo YS, Yoo JL, Lee G. Pathologic differences between placentas from intrauterine growth restriction pregnancies with and without absent or reversed end diastolic velocity of umbilical arteries. Korean J Pathol. 2011; 45:36–44.
Article14. Park SY, Kim MY, Kim YJ, et al. Placental pathology in intrauterine growth retardation. Korean J Pathol. 2002; 36:30–7.15. Trowsdale J, Betz AG. Mother’s little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol. 2006; 7:241–6.
Article16. Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol. 2013; 13:23–33.
Article17. Kim MJ, Romero R, Kim CJ, et al. Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the feto-maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease. J Immunol. 2009; 182:3919–27.
Article18. Lee J, Romero R, Xu Y, et al. Maternal HLA panel-reactive antibodies in early gestation positively correlate with chronic chorioamnionitis: evidence in support of the chronic nature of maternal anti-fetal rejection. Am J Reprod Immunol. 2011; 66:510–26.
Article19. Lee J, Romero R, Xu Y, et al. A signature of maternal anti-fetal rejection in spontaneous preterm birth: chronic chorioamnionitis, anti-human leukocyte antigen antibodies, and C4d. PLoS One. 2011; 6:e16806.
Article20. Perni SC, Predanic M, Cho JE, Baergen RN. Placental pathology and pregnancy outcomes in donor and non-donor oocyte in vitro fertilization pregnancies. J Perinat Med. 2005; 33:27–32.
Article21. Gundogan F, Bianchi DW, Scherjon SA, Roberts DJ. Placental pathology in egg donor pregnancies. Fertil Steril. 2010; 93:397–404.
Article22. Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015; 64:1–65.23. Eberle AM, Levesque D, Vintzileos AM, Egan JF, Tsapanos V, Salafia CM. Placental pathology in discordant twins. Am J Obstet Gynecol. 1993; 169:931–5.
Article24. Jacques SM, Qureshi F. Chronic villitis of unknown etiology in twin gestations. Pediatr Pathol. 1994; 14:575–84.
Article25. Yusuf K, Kliman HJ. The fetus, not the mother, elicits maternal immunologic rejection: lessons from discordant dizygotic twin placentas. J Perinat Med. 2008; 36:291–6.
Article26. Redline RW. Villitis of unknown etiology: noninfectious chronic villitis in the placenta. Hum Pathol. 2007; 38:1439–46.
Article27. Altshuler G, Russell P. The human placental villitides: a review of chronic intrauterine infection. Curr Top Pathol. 1975; 60:64–112.
Article28. Becroft DM, Thompson JM, Mitchell EA. Placental villitis of unknown origin: epidemiologic associations. Am J Obstet Gynecol. 2005; 192:264–71.
Article29. Redline RW, Patterson P. Villitis of unknown etiology is associated with major infiltration of fetal tissue by maternal inflammatory cells. Am J Pathol. 1993; 143:473–9.30. Knox WF, Fox H. Villitis of unknown aetiology: its incidence and significance in placentae from a British population. Placenta. 1984; 5:395–402.
Article31. Redline RW, Patterson P. Patterns of placental injury. Correlations with gestational age, placental weight, and clinical diagnoses. Arch Pathol Lab Med. 1994; 118:698–701.32. Russell P. Inflammatory lesions of the human placenta. III. The histopathology of villitis of unknown aetiology. Placenta. 1980; 1:227–44.
Article33. Breathnach FM, Malone FD. Fetal growth disorders in twin gestations. Semin Perinatol. 2012; 36:175–81.
Article