J Bone Metab.
2017 May;24(2):75-82. 10.11005/jbm.2017.24.2.75.
Transcriptional Network Controlling Endochondral Ossification
- Affiliations
-
- 1Department of Molecular and Cellular Biochemistry, Osaka University Graduate School of Dentistry, Osaka, Japan. hata@dent.osaka-u.ac.jp
- KMID: 2379988
- DOI: http://doi.org/10.11005/jbm.2017.24.2.75
Abstract
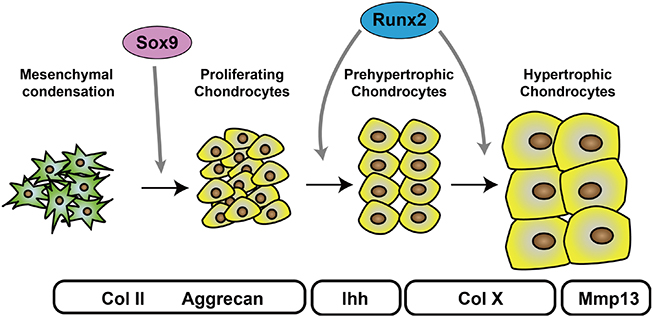
- Endochondral ossification is the fundamental process of skeletal development in vertebrates. Chondrocytes undergo sequential steps of differentiation, including mesenchymal condensation, proliferation, hypertrophy, and mineralization. These steps, which are required for the morphological and functional changes in differentiating chondrocytes, are strictly regulated by a complex transcriptional network. Biochemical and mice genetic studies identified chondrogenic transcription factors critical for endochondral ossification. The transcription factor sex-determining region Y (SRY)-box 9 (Sox9) is essential for early chondrogenesis, and impaired Sox9 function causes severe chondrodysplasia in humans and mice. In addition, recent genome-wide chromatin immunoprecipitation-sequencing studies revealed the precise regulatory mechanism of Sox9 during early chondrogenesis. Runt-related transcription factor 2 promotes chondrocyte hypertrophy and terminal differentiation. Interestingly, endoplasmic reticulum (ER) stress-related transcription factors have recently emerged as novel regulators of chondrocyte differentiation. Here we review the transcriptional mechanisms that regulate endochondral ossification, with a focus on Sox9.
MeSH Terms
Figure
Cited by 2 articles
-
Regulation of Cartilage Development and Diseases by Transcription Factors
Riko Nishimura, Kenji Hata, Yoshifumi Takahata, Tomohiko Murakami, Eriko Nakamura, Hiroko Yagi
J Bone Metab. 2017;24(3):147-153. doi: 10.11005/jbm.2017.24.3.147.Ankylosing Neurogenic Myositis Ossificans of the Hip: A Case Series and Review of Literature
Byung-Ho Yoon, In Keun Park, Yerl-Bo Sung
Hip Pelvis. 2018;30(2):86-91. doi: 10.5371/hp.2018.30.2.86.
Reference
-
1. Long F, Ornitz DM. Development of the endochondral skeleton. Cold Spring Harb Perspect Biol. 2013; 5:a008334.
Article2. Capdevila J, Johnson RL. Endogenous and ectopic expression of noggin suggests a conserved mechanism for regulation of BMP function during limb and somite patterning. Dev Biol. 1998; 197:205–217.
Article3. Bandyopadhyay A, Tsuji K, Cox K, et al. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genet. 2006; 2:e216.
Article4. Yu K, Ornitz DM. FGF signaling regulates mesenchymal differentiation and skeletal patterning along the limb bud proximodistal axis. Development. 2008; 135:483–491.
Article5. Yu K, Xu J, Liu Z, et al. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development. 2003; 130:3063–3074.
Article6. Ornitz DM, Legeai-Mallet L. Achondroplasia: development, pathogenesis, and therapy. Dev Dyn. 2017; 246:291–309.
Article7. Foster JW, Dominguez-Steglich MA, Guioli S, et al. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994; 372:525–530.
Article8. Wagner T, Wirth J, Meyer J, et al. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994; 79:1111–1120.
Article9. Wright E, Hargrave MR, Christiansen J, et al. The Sry-related gene Sox9 is expressed during chondrogenesis in mouse embryos. Nat Genet. 1995; 9:15–20.
Article10. Bell DM, Leung KK, Wheatley SC, et al. SOX9 directly regulates the type-II collagen gene. Nat Genet. 1997; 16:174–178.11. Lefebvre V, Huang W, Harley VR, et al. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol Cell Biol. 1997; 17:2336–2346.
Article12. Ng LJ, Wheatley S, Muscat GE, et al. SOX9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Dev Biol. 1997; 183:108–121.
Article13. Zhou G, Lefebvre V, Zhang Z, et al. Three high mobility group-like sequences within a 48-base pair enhancer of the Col2a1 gene are required for cartilage-specific expression in vivo. J Biol Chem. 1998; 273:14989–14997.
Article14. Bi W, Deng JM, Zhang Z, et al. Sox9 is required for cartilage formation. Nat Genet. 1999; 22:85–89.
Article15. Bi W, Huang W, Whitworth DJ, et al. Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc Natl Acad Sci U S A. 2001; 98:6698–6703.
Article16. Akiyama H, Chaboissier MC, Martin JF, et al. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002; 16:2813–2828.
Article17. Mori-Akiyama Y, Akiyama H, Rowitch DH, et al. Sox9 is required for determination of the chondrogenic cell lineage in the cranial neural crest. Proc Natl Acad Sci U S A. 2003; 100:9360–9365.
Article18. Akiyama H, Stadler HS, Martin JF, et al. Misexpression of Sox9 in mouse limb bud mesenchyme induces polydactyly and rescues hypodactyly mice. Matrix Biol. 2007; 26:224–233.
Article19. Lefebvre V, Zhou G, Mukhopadhyay K, et al. An 18-base-pair sequence in the mouse proalpha1(II) collagen gene is sufficient for expression in cartilage and binds nuclear proteins that are selectively expressed in chondrocytes. Mol Cell Biol. 1996; 16:4512–4523.
Article20. Liu Y, Li H, Tanaka K, et al. Identification of an enhancer sequence within the first intron required for cartilage-specific transcription of the alpha2(XI) collagen gene. J Biol Chem. 2000; 275:12712–12718.
Article21. Lefebvre V, Li P, de Crombrugghe B. A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. EMBO J. 1998; 17:5718–5733.
Article22. Smits P, Dy P, Mitra S, et al. Sox5 and Sox6 are needed to develop and maintain source, columnar, and hypertrophic chondrocytes in the cartilage growth plate. J Cell Biol. 2004; 164:747–758.
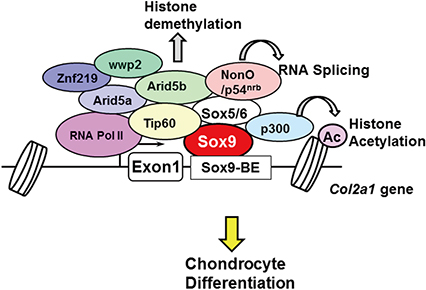
Article23. Amano K, Hata K, Muramatsu S, et al. Arid5a cooperates with Sox9 to stimulate chondrocyte-specific transcription. Mol Biol Cell. 2011; 22:1300–1311.
Article24. Hata K, Nishimura R, Muramatsu S, et al. Paraspeckle protein p54nrb links Sox9-mediated transcription with RNA processing during chondrogenesis in mice. J Clin Invest. 2008; 118:3098–3108.
Article25. Takigawa Y, Hata K, Muramatsu S, et al. The transcription factor Znf219 regulates chondrocyte differentiation by assembling a transcription factory with Sox9. J Cell Sci. 2010; 123:3780–3788.
Article26. Hata K, Takashima R, Amano K, et al. Arid5b facilitates chondrogenesis by recruiting the histone demethylase Phf2 to Sox9-regulated genes. Nat Commun. 2013; 4:2850.
Article27. Hattori T, Coustry F, Stephens S, et al. Transcriptional regulation of chondrogenesis by coactivator Tip60 via chromatin association with Sox9 and Sox5. Nucleic Acids Res. 2008; 36:3011–3024.
Article28. Akiyama H, Lyons JP, Mori-Akiyama Y, et al. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004; 18:1072–1087.
Article29. Tsuda M, Takahashi S, Takahashi Y, et al. Transcriptional coactivators CREB-binding protein and p300 regulate chondrocyte-specific gene expression via association with Sox9. J Biol Chem. 2003; 278:27224–27229.
Article30. Zhou R, Bonneaud N, Yuan CX, et al. SOX9 interacts with a component of the human thyroid hormone receptor-associated protein complex. Nucleic Acids Res. 2002; 30:3245–3252.
Article31. Nakamura Y, Yamamoto K, He X, et al. Wwp2 is essential for palatogenesis mediated by the interaction between Sox9 and mediator subunit 25. Nat Commun. 2011; 2:251.
Article32. Kawakami Y, Tsuda M, Takahashi S, et al. Transcriptional coactivator PGC-1alpha regulates chondrogenesis via association with Sox9. Proc Natl Acad Sci U S A. 2005; 102:2414–2419.
Article33. Liu CF, Lefebvre V. The transcription factors SOX9 and SOX5/SOX6 cooperate genome-wide through super-enhancers to drive chondrogenesis. Nucleic Acids Res. 2015; 43:8183–8203.
Article34. Ohba S, He X, Hojo H, et al. Distinct transcriptional programs underlie Sox9 regulation of the mammalian chondrocyte. Cell Rep. 2015; 12:229–243.
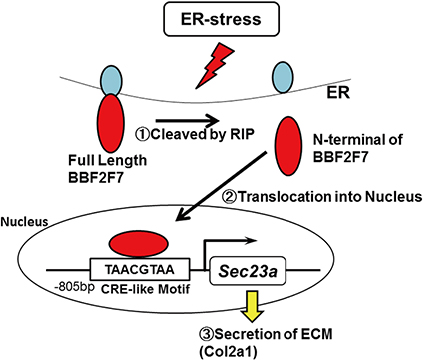
Article35. Saito A, Hino S, Murakami T, et al. Regulation of endoplasmic reticulum stress response by a BBF2H7-mediated Sec23a pathway is essential for chondrogenesis. Nat Cell Biol. 2009; 11:1197–1204.
Article36. Wang W, Lian N, Li L, et al. Atf4 regulates chondrocyte proliferation and differentiation during endochondral ossification by activating Ihh transcription. Development. 2009; 136:4143–4153.
Article37. Farnum CE, Lee R, O'Hara K, et al. Volume increase in growth plate chondrocytes during hypertrophy: the contribution of organic osmolytes. Bone. 2002; 30:574–581.
Article38. Cooper KL, Oh S, Sung Y, et al. Multiple phases of chondrocyte enlargement underlie differences in skeletal proportions. Nature. 2013; 495:375–378.
Article39. Ducy P, Zhang R, Geoffroy V, et al. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997; 89:747–754.
Article40. Takeda S, Bonnamy JP, Owen MJ, et al. Continuous expression of Cbfa1 in nonhypertrophic chondrocytes uncovers its ability to induce hypertrophic chondrocyte differentiation and partially rescues Cbfa1-deficient mice. Genes Dev. 2001; 15:467–481.
Article41. Inada M, Yasui T, Nomura S, et al. Maturational disturbance of chondrocytes in Cbfa1-deficient mice. Dev Dyn. 1999; 214:279–290.42. Yoshida CA, Yamamoto H, Fujita T, et al. Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Genes Dev. 2004; 18:952–963.
Article43. Li F, Lu Y, Ding M, et al. Runx2 contributes to murine Col10a1 gene regulation through direct interaction with its cis-enhancer. J Bone Miner Res. 2011; 26:2899–2910.
Article44. Hirata M, Kugimiya F, Fukai A, et al. C/EBPbeta and RUNX2 cooperate to degrade cartilage with MMP-13 as the target and HIF-2alpha as the inducer in chondrocytes. Hum Mol Genet. 2012; 21:1111–1123.
Article45. Arnold MA, Kim Y, Czubryt MP, et al. MEF2C transcription factor controls chondrocyte hypertrophy and bone development. Dev Cell. 2007; 12:377–389.
Article46. Verzi MP, Agarwal P, Brown C, et al. The transcription factor MEF2C is required for craniofacial development. Dev Cell. 2007; 12:645–652.
Article47. Ionescu A, Kozhemyakina E, Nicolae C, et al. FoxA family members are crucial regulators of the hypertrophic chondrocyte differentiation program. Dev Cell. 2012; 22:927–939.
Article48. Yoshida M, Hata K, Takashima R, et al. The transcription factor Foxc1 is necessary for Ihh-Gli2-regulated endochondral ossification. Nat Commun. 2015; 6:6653.
Article49. He X, Ohba S, Hojo H, et al. AP-1 family members act with Sox9 to promote chondrocyte hypertrophy. Development. 2016; 143:3012–3023.
Article50. Dy P, Wang W, Bhattaram P, et al. Sox9 directs hypertrophic maturation and blocks osteoblast differentiation of growth plate chondrocytes. Dev Cell. 2012; 22:597–609.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- NOX4 and its association with Anatomy/Histology/ Embryology myeloperoxidase and osteopontin in regulating endochondral ossification
- Delayed Endochondral Ossification in Hsp70 Knock-out Mice Fetuses due to Maternal Hyperthermia
- A Case of Thanatophoric Dysplasia Type II Prenatally Diagnosed at 20 Gestational Weeks
- Endometrial Ossification: Clinical and pathological analysis of 7 cases
- Expression patterns of beta ig-h3 in chondrocyte differentiation during endochondral ossification