Allergy Asthma Respir Dis.
2017 May;5(3):147-152. 10.4168/aard.2017.5.3.147.
Clinical implication of exhaled breath temperature measurement in pediatric asthma
- Affiliations
-
- 1Department of Pediatrics, Institute of Allergy, Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea. YHKIM@yuhs.ac
- KMID: 2379942
- DOI: http://doi.org/10.4168/aard.2017.5.3.147
Abstract
- PURPOSE
Exhaled breath temperature (EBT) has been suggested as a noninvasive marker of airway inflammation in asthma. The aim of this study was to determine its clinical implication in children with asthma.
METHODS
A total of 233 children were enrolled in this study. Among them, 116 were asthmatic children and 117 were healthy children. Spirometry, bronchodilator response (BDR) test, methacholine challenge test, and skin prick test were performed. EBT, fractional exhaled nitric oxide (FeNO), blood eosinophils, and total IgE levels were measured. EBT was measured by using X-halo.
RESULTS
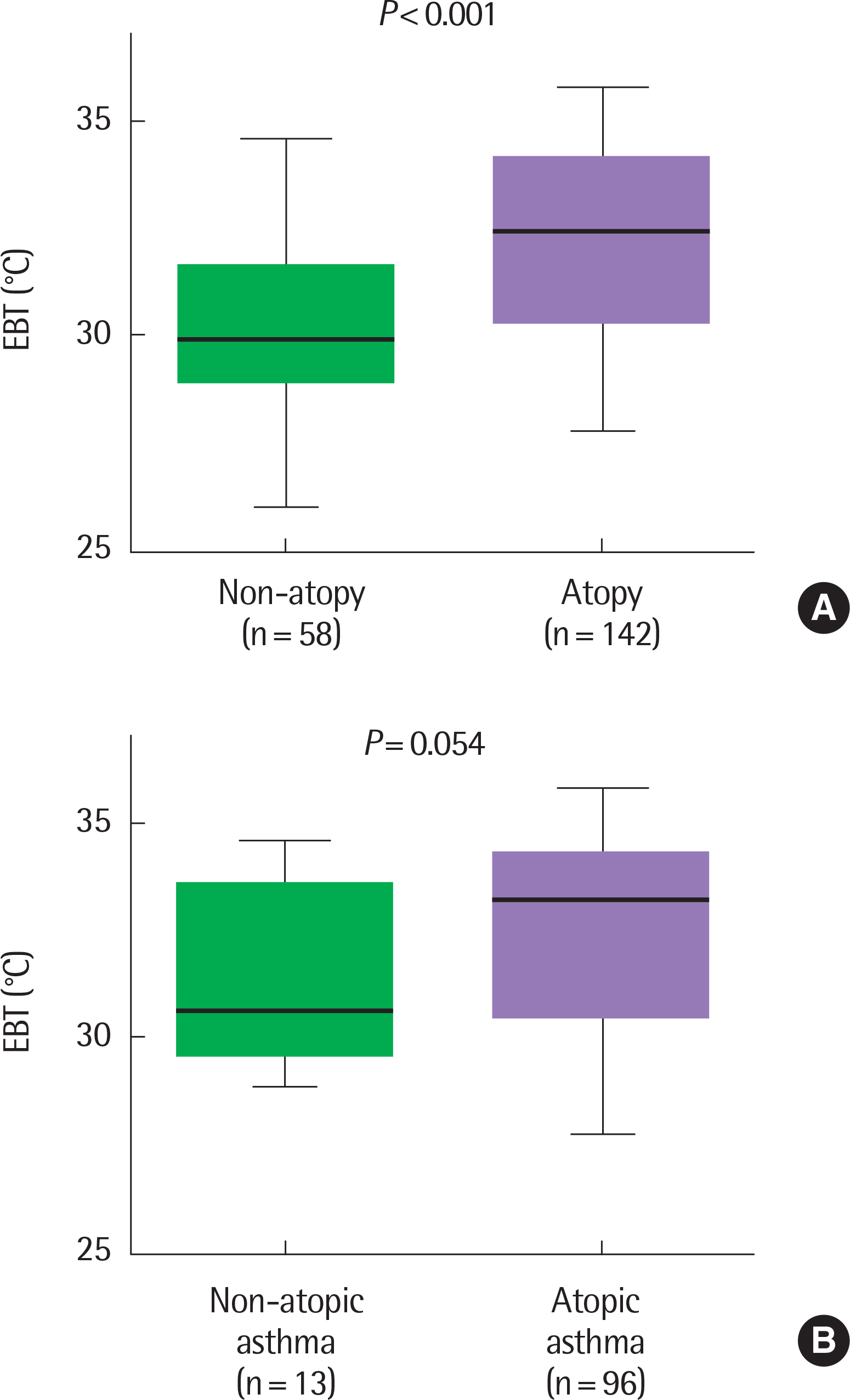
EBT was significantly higher in the asthma group than in the control group (median [interquartile range], 32.1℃ [30.0℃-33.9℃] vs. 29.7℃ [29.0℃-31.3℃], P<0.001). EBT was significantly higher in poorly or partly controlled asthmatic children than well-controlled asthmatic children (33.5℃ [31.0℃-34.4℃] vs. 30.3℃ [29.3℃-32.9℃], P<0.0001). Among total subjects, EBT was significantly higher in the atopic group than in the nonatopic group (32.4℃ [30.3℃-34.0℃] vs. 29.8℃ [29.0℃-30.3℃], P<0.001). There were neither significant associations between EBT and BDR (r=0.109, P=0.241) nor between EBT and PC20 (provocation concentration causing a 20% fall in FEV1) in total subjects (r=0.127, P=0.316). EBT did not show any association with FeNO (r=0.353, P=0.071).
CONCLUSION
Our study suggests that EBT might play a role as an ancillary marker for allergic airway inflammation and the degree of control in pediatric asthma patients. Additional studies are required to explore the value of EBT in detail.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Could exhaled breath temperature discriminate the asthma?
Man Yong Han
Allergy Asthma Respir Dis. 2017;5(3):121-122. doi: 10.4168/aard.2017.5.3.121.
Reference
-
1. Bacharier LB, Boner A, Carlsen KH, Eigenmann PA, Frischer T, Götz M, et al. Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy. 2008; 63:5–34.
Article2. Vignola AM, Mirabella F, Costanzo G, Di Giorgi R, Gjomarkaj M, Bellia V, et al. Airway remodeling in asthma. Chest. 2003; 123(3 Suppl):417S–422S.
Article3. Svensson H, Nilsson D, Bjermer L, Tufvesson E. Exhaled breath temperature increases after exercise in asthmatics and controls. Respiration. 2012; 84:283–90.
Article4. Djukanović R, Roche WR, Wilson JW, Beasley CR, Twentyman OP, Howarth RH, et al. Mucosal inflammation in asthma. Am Rev Respir Dis. 1990; 142:434–57.
Article5. Busse WW. Inflammation in asthma: the cornerstone of the disease and target of therapy. J Allergy Clin Immunol. 1998; 102(4 Pt 2):S17–22.
Article6. Melo RE, Popov TA, Solé D. Exhaled breath temperature, a new bio-marker in asthma control: a pilot study. J Bras Pneumol. 2010; 36:693–9.7. Popov TA, Dunev S, Kralimarkova TZ, Kraeva S, DuBuske LM. Evaluation of a simple, potentially individual device for exhaled breath temperature measurement. Respir Med. 2007; 101:2044–50.
Article8. Fahy JV. Eosinophilic and neutrophilic inflammation in asthma: insights from clinical studies. Proc Am Thorac Soc. 2009; 6:256–9.
Article9. Pizzichini MM, Popov TA, Efthimiadis A, Hussack P, Evans S, Pizzichini E, et al. Spontaneous and induced sputum to measure indices of airway inflammation in asthma. Am J Respir Crit Care Med. 1996; 154(4 Pt 1):866–9.
Article10. American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005; 171:912–30.11. Piacentini GL, Bodini A, Zerman L, Costella S, Zanolla L, Peroni DG, et al. Relationship between exhaled air temperature and exhaled nitric oxide in childhood asthma. Eur Respir J. 2002; 20:108–11.
Article12. Covar RA, Spahn JD, Martin RJ, Silkoff PE, Sundstrom DA, Murphy J, et al. Safety and application of induced sputum analysis in childhood asthma. J Allergy Clin Immunol. 2004; 114:575–82.
Article13. van Rensen EL, Straathof KC, Veselic-Charvat MA, Zwinderman AH, Bel EH, Sterk PJ. Effect of inhaled steroids on airway hyperresponsiveness, sputum eosinophils, and exhaled nitric oxide levels in patients with asthma. Thorax. 1999; 54:403–8.
Article14. Kharitonov SA, Barnes PJ. Exhaled biomarkers. Chest. 2006; 130:1541–6.
Article15. Logie KM, Kusel MM, Sly PD, Hall GL. Exhaled breath temperature in healthy children is influenced by room temperature and lung volume. Pediatr Pulmonol. 2011; 46:1062–8.
Article16. Paredi P, Kharitonov SA, Barnes PJ. Faster rise of exhaled breath temperature in asthma: a novel marker of airway inflammation? Am J Respir Crit Care Med. 2002; 165:181–4.17. Piacentini GL, Peroni D, Crestani E, Zardini F, Bodini A, Costella S, et al. Exhaled air temperature in asthma: methods and relationship with markers of disease. Clin Exp Allergy. 2007; 37:415–9.
Article18. Piacentini GL, Bodini A, Peroni D, Ress M, Costella S, Boner AL. Exhaled air temperature and eosinophil airway inflammation in allergic asthmatic children. J Allergy Clin Immunol. 2004; 114:202–4.
Article19. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, November 1986. Am Rev Respir Dis. 1987; 136:225–44.20. Levy ML, Hardwell A, McKnight E, Holmes J. Asthma patients' inability to use a pressurised metered-dose inhaler (pMDI) correctly correlates with poor asthma control as defined by the global initiative for asthma (GINA) strategy: a retrospective analysis. Prim Care Respir J. 2013; 22:406–11.
Article21. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005; 26:319–38.22. Brown WG, Halonen MJ, Kaltenborn WT, Barbee RA. The relationship of respiratory allergy, skin test reactivity, and serum IgE in a community population sample. J Allergy Clin Immunol. 1979; 63:328–35.
Article23. Kim YH, Kim KW, Baek J, Park HB, Kim H, Song KJ, et al. Usefulness of impulse oscillometry and fractional exhaled nitric oxide in children with Eosinophilic bronchitis. Pediatr Pulmonol. 2013; 48:221–8.
Article24. Vints AM, Oostveen E, Eeckhaut G, Smolders M, De Backer WA. Time-dependent effect of nitrate-rich meals on exhaled nitric oxide in healthy subjects. Chest. 2005; 128:2465–70.
Article25. Salvato G. Quantitative and morphological analysis of the vascular bed in bronchial biopsy specimens from asthmatic and nonasthmatic subjects. Thorax. 2001; 56:902–6.
Article26. Bailey SR, Boustany S, Burgess JK, Hirst SJ, Sharma HS, Simcock DE, et al. Airway vascular reactivity and vascularisation in human chronic airway disease. Pulm Pharmacol Ther. 2009; 22:417–25.
Article27. Xepapadaki P, Xatziioannou A, Chatzicharalambous M, Makrinioti H, Papadopoulos NG. Exhaled breath temperature increases during mild exacerbations in children with virus-induced asthma. Int Arch Allergy Immunol. 2010; 153:70–4.
Article28. Paredi P, Kharitonov SA, Barnes PJ. Correlation of exhaled breath temperature with bronchial blood flow in asthma. Respir Res. 2005; 6:15.
Article29. Kumar SD, Emery MJ, Atkins ND, Danta I, Wanner A. Airway mucosal blood flow in bronchial asthma. Am J Respir Crit Care Med. 1998; 158:153–6.
Article30. Peroni DG, Chinellato I, Piazza M, Zardini F, Bodini A, Olivieri F, et al. Exhaled breath temperature and exercise-induced bronchoconstriction in asthmatic children. Pediatr Pulmonol. 2012; 47:240–4.
Article31. Robbins RA, Springall DR, Warren JB, Kwon OJ, Buttery LD, Wilson AJ, et al. Inducible nitric oxide synthase is increased in murine lung epithelial cells by cytokine stimulation. Biochem Biophys Res Commun. 1994; 198:835–43.
Article32. Fagan KA, Tyler RC, Sato K, Fouty BW, Morris KG Jr, Huang PL, et al. Relative contributions of endothelial, inducible, and neuronal NOS to tone in the murine pulmonary circulation. Am J Physiol. 1999; 277(3 Pt 1):L472–8.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Could exhaled breath temperature discriminate the asthma?
- Measurements of fractional exhaled nitric oxide in pediatric asthma
- Use of the exhaled nitric oxide for management of asthma and respiratory diseases
- Analysis of clinical features of adult asthma according to blood basophils and their association with cytokines in exhaled breath condensate
- Biomarkers for Recurrent Wheezing and Asthma in Preschool Children