Chonnam Med J.
2017 May;53(2):118-126. 10.4068/cmj.2017.53.2.118.
Ameliorative Effects of Nilotinib on CCl4 Induced Liver Fibrosis Via Attenuation of RAGE/HMGB1 Gene Expression and Oxidative Stress in Rat
- Affiliations
-
- 1Department of Biochemistry, School of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran. jamshidkarimi2013@gmail.com
- 2Department of Immunology, School of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran.
- 3Department of Pathobiology, Veterinary Medicine Faculty, Razi University, Kermanshah, Iran.
- KMID: 2379284
- DOI: http://doi.org/10.4068/cmj.2017.53.2.118
Abstract
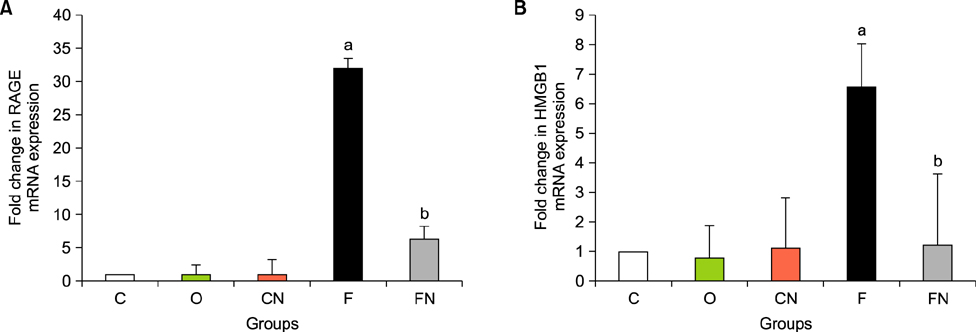
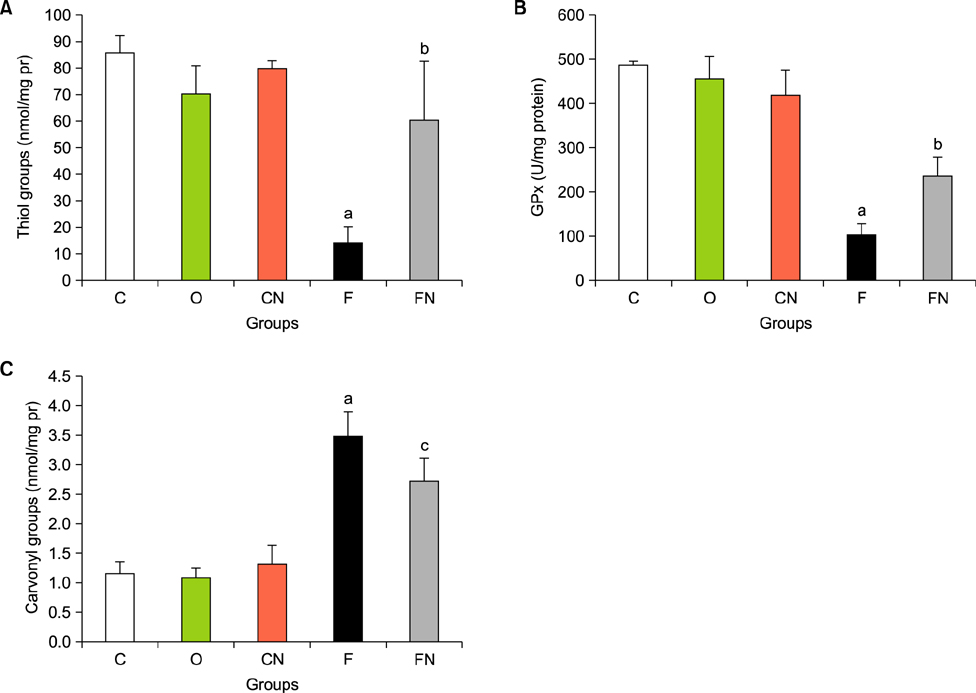
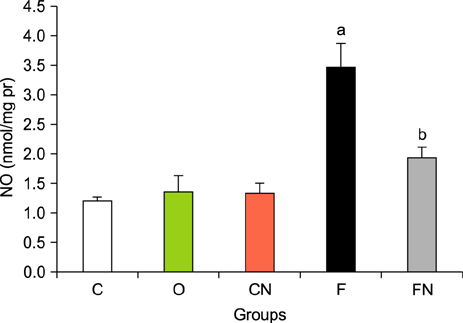
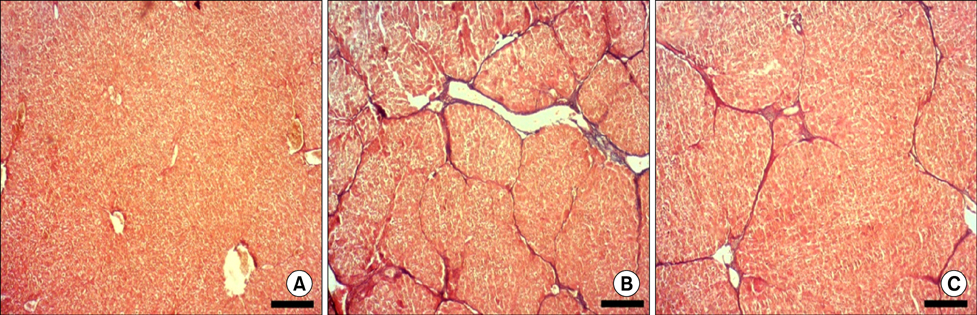
- Nilotinib as a tyrosine kinase inhibitor has been recently used to improve the liver fibrosis process, but the exact mechanisms still require further clarification. In this study, we investigated the anti-fibrotic effects of Nilotinib via RAGE/HMGB1axis and antioxidant mechanisms. This experimental study was performed in the Hamadan University of Medical Sciences, Iran, from May 2015 to December 2016. Liver fibrosis was induced in Wistar male rats by CCL₄. Rats were gavaged daily with Nilotinib (10 mg/kg). RAGE, HMGB1, TNF-α and TGF-β mRNA expression were evaluated by quantitative RT-PCR. TNF-α protein levels were measured using the immunoassay method. Thiol groups, carbonyl groups, nitric oxide levels and glutathione peroxidase activity were measured by spectrophotometric methods.The results showed that Nilotinib decreased TNF-α, TGF-β, RAGE and HMGB1 mRNA expression (p<0.001) in the liver tissues of the fibrosis group. Nilotinib also decreased carbonyl groups and nitric oxide levels and increased thiol groups and glutathione peroxidase activity in the fibrosis groups. The histopathological changes were found to be attenuated by Nilotinib. In conclusion, Nilotinib can improve liver fibrosis and open new mechanisms of the anti-fibrotic properties of Nilotinib.
Keyword
MeSH Terms
Figure
Reference
-
1. Poynard T, Lebray P, Ingiliz P, Varaut A, Varsat B, Ngo Y, et al. Prevalence of liver fibrosis and risk factors in a general population using non-invasive biomarkers (FibroTest). BMC Gastroenterol. 2010; 10:40.
Article2. Wang P, Koyama Y, Liu X, Xu J, Ma HY, Liang S, et al. Promising therapy candidates for liver fibrosis. Front Physiol. 2016; 7:47.
Article3. Hauff P, Gottwald U, Ocker M. Early to Phase II drugs currently under investigation for the treatment of liver fibrosis. Expert Opin Investig Drugs. 2015; 24:309–327.
Article4. Seki E, Brenner DA. Recent advancement of molecular mechanisms of liver fibrosis. J Hepatobiliary Pancreat Sci. 2015; 22:512–518.
Article5. Khazaei M, Karimi J, Sheikh N, Goodarzi MT, Saidijam M, Khodadadi I, et al. Effects of Resveratrol on Receptor for Advanced Glycation End Products (RAGE) expression and oxidative stress in the liver of rats with type 2 diabetes. Phytother Res. 2016; 30:66–71.
Article6. Sorci G, Riuzzi F, Giambanco I, Donato R. RAGE in tissue homeostasis, repair and regeneration. Biochim Biophys Acta. 2013; 1833:101–109.
Article7. Ramasamy R, Yan SF, Schmidt AM. The diverse ligand repertoire of the receptor for advanced glycation endproducts and pathways to the complications of diabetes. Vascul Pharmacol. 2012; 57:160–167.
Article8. Basta G, Navarra T, De Simone P, Del Turco S, Gastaldelli A, Filipponi F. What is the role of the receptor for advanced glycation end products-ligand axis in liver injury? Liver Transpl. 2011; 17:633–640.
Article9. Fehrenbach H, Weiskirchen R, Kasper M, Gressner AM. Up-regulated expression of the receptor for advanced glycation end products in cultured rat hepatic stellate cells during transdifferentiation to myofibroblasts. Hepatology. 2001; 34:943–952.
Article10. Lohwasser C, Neureiter D, Popov Y, Bauer M, Schuppan D. Role of the receptor for advanced glycation end products in hepatic fibrosis. World J Gastroenterol. 2009; 15:5789–5798.
Article11. Qu K, Huang Z, Lin T, Liu S, Chang H, Yan Z, et al. New Insight into the anti-liver fibrosis effect of multitargeted tyrosine kinase inhibitors: from molecular target to clinical trials. Front Pharmacol. 2016; 6:300.
Article12. Shiha GE, Abu-Elsaad NM, Zalata KR, Ibrahim TM. Tracking anti-fibrotic pathways of nilotinib and imatinib in experimentally induced liver fibrosis: an insight. Clin Exp Pharmacol Physiol. 2014; 41:788–797.
Article13. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72:248–254.
Article14. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25:402–408.
Article15. Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990; 186:464–478.16. Hu ML. Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol. 1994; 233:380–385.17. Saneyasu T, Akhtar R, Sakai T. Molecular Cues Guiding Matrix Stiffness in Liver Fibrosis. Biomed Res Int. 2016; 2016:2646212.
Article18. Friedman SL. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol. 2010; 7:425–436.
Article19. Liu Y, Wang Z, Kwong SQ, Lui EL, Friedman SL, Li FR, et al. Inhibition of PDGF, TGF-XMLLink_XYZ, and Abl signaling and reduction of liver fibrosis by the small molecule Bcr-Abl tyrosine kinase antagonist Nilotinib. J Hepatol. 2011; 55:612–625.
Article20. Zeng S, Feirt N, Goldstein M, Guarrera J, Ippagunta N, Ekong U, et al. Blockade of receptor for advanced glycation end product (RAGE) attenuates ischemia and reperfusion injury to the liver in mice. Hepatology. 2004; 39:422–432.
Article21. Ekong U, Zeng S, Dun H, Feirt N, Guo J, Ippagunta N, et al. Blockade of the receptor for advanced glycation end products attenuates acetaminophen-induced hepatotoxicity in mice. J Gastroenterol Hepatol. 2006; 21:682–688.
Article22. Xia JR, Liu NF, Zhu NX. Specific siRNA targeting the receptor for advanced glycation end products inhibits experimental hepatic fibrosis in rats. Int J Mol Sci. 2008; 9:638–661.
Article23. Kao YH, Jawan B, Goto S, Hung CT, Lin YC, Nakano T, et al. High-mobility group box 1 protein activates hepatic stellate cells in vitro. Transplant Proc. 2008; 40:2704–2705.
Article24. Ge WS, Wu JX, Fan JG, Wang YJ, Chen YW. Inhibition of high-mobility group box 1 expression by siRNA in rat hepatic stellate cells. World J Gastroenterol. 2011; 17:4090–4098.
Article25. Arriazu E, Ge X, Leung TM, Magdaleno F, Lopategi A, Lu Y, et al. Signalling via the osteopontin and high mobility group box-1 axis drives the fibrogenic response to liver injury. Gut. 2016; DOI: 10.1136/gutjnl-2015-310752. [Epub ahead of print].
Article26. Ge X, Antoine DJ, Lu Y, Arriazu E, Leung TM, Klepper AL, et al. High mobility group box-1 (HMGB1) participates in the pathogenesis of alcoholic liver disease (ALD). J Biol Chem. 2014; 289:22672–22691.
Article27. Mohammadalipour A, Karimi J, Khodadadi I, Solgi G, Hashemnia M, Sheikh N, et al. Dasatinib prevent hepatic fibrosis induced by carbon tetrachloride (CCl4) via anti-inflammatory and antioxidant mechanism. Immunopharmacol Immunotoxicol. 2017; 39:19–27.
Article28. Sánchez-Valle V, Chávez-Tapia NC, Uribe M, Méndez-Sánchez N. Role of oxidative stress and molecular changes in liver fibrosis: a review. Curr Med Chem. 2012; 19:4850–4860.
Article29. Shaker ME. Nilotinib interferes with the signalling pathways implicated in acetaminophen hepatotoxicity. Basic Clin Pharmacol Toxicol. 2014; 114:263–270.
Article30. Diesen DL, Kuo PC. Nitric oxide and redox regulation in the liver: Part I. General considerations and redox biology in hepatitis. J Surg Res. 2010; 162:95–109.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- HMGB1 increases RAGE expression in vascular smooth muscle cells via ERK and p-38 MAPK-dependent pathways
- Inhibitory Effect of Tetrandrine on Extracellular Matrix Deposition in Rat Hepatic Fibrosis
- Current Understanding of HMGB1-mediated Autophagy
- The Change of Transforming Growth Factor-beta1 Expression and the Effect of Vitamin E in the Acute Rat Liver Injury with Carbon Tetrachloride
- The Effects of CCl4 on the Expressions of Aquaporin and Superoxide Dismutase in the Kidney of the Spontaneously Hypertensive Rat