Korean J Neurotrauma.
2015 Oct;11(2):44-51. 10.13004/kjnt.2015.11.2.44.
Time Course and Characteristics of Astrocyte Activation in the Rat Brain after Injury
- Affiliations
-
- 1Department of Neurosurgery, Myongji Hospital, Goyang, Korea. psc710@mjh.or.kr
- KMID: 2378257
- DOI: http://doi.org/10.13004/kjnt.2015.11.2.44
Abstract
OBJECTIVE
After injury to the central nervous system (CNS), glial scar tissue is formed in the process of wound healing. This can be is a clinical problem because it interferes with axonal regeneration and functional recovery. It is known that intracellular proteins, including the glial fibrillary acidic protein (GFAP), nestin, and vimentin increase in the astrocytes after an injury to the CNS. By studying the time course and co-expression pattern of these intracellular proteins, this study will attempt to prove that these proteins are involved in the processes of glial scar formation.
METHODS
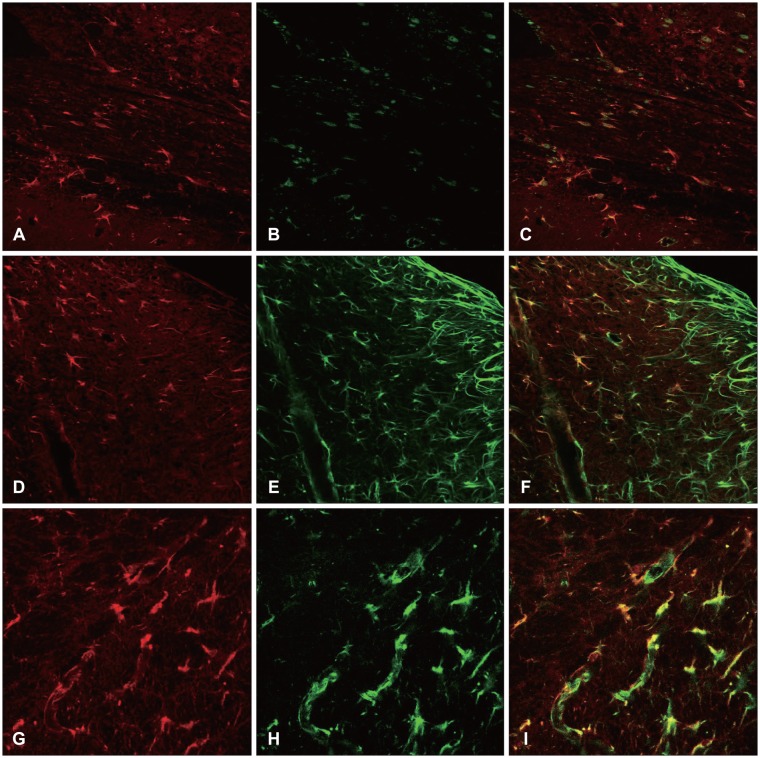
Twenty-five male Sprague-Dawley rats were used in this study. Bregma of the cerebral cortex, an area was incised with a sharp blade, and perfusion was performed. The expressions of the intracellular proteins were assayed, while the co-localization of the intermediate filament (GFAP, nestin, and vimentin) and A2B5 were examined.
RESULTS
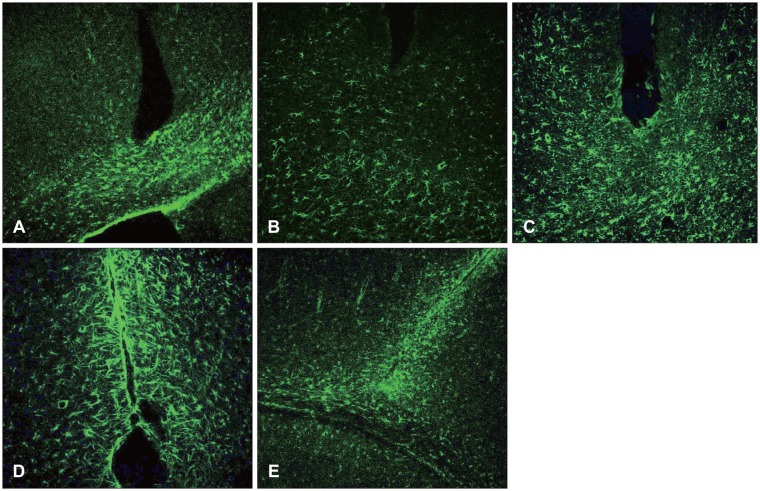
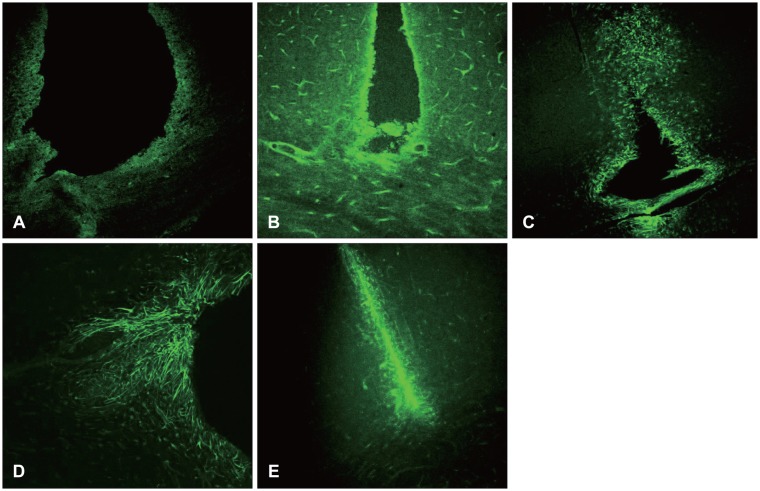
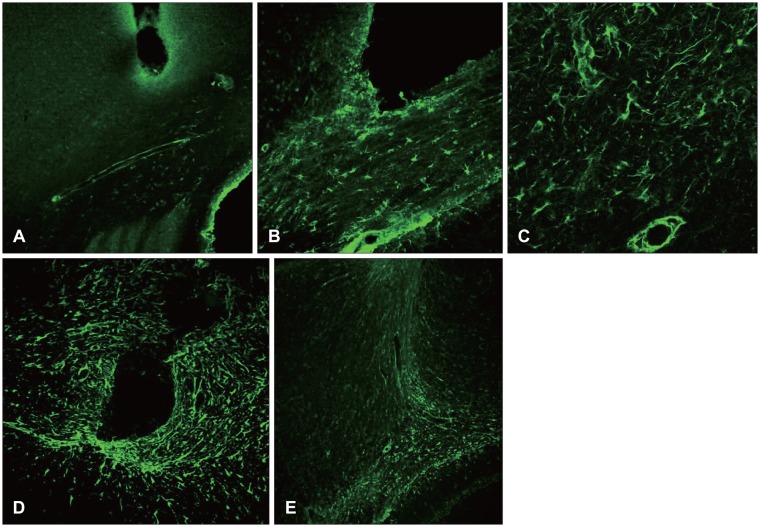
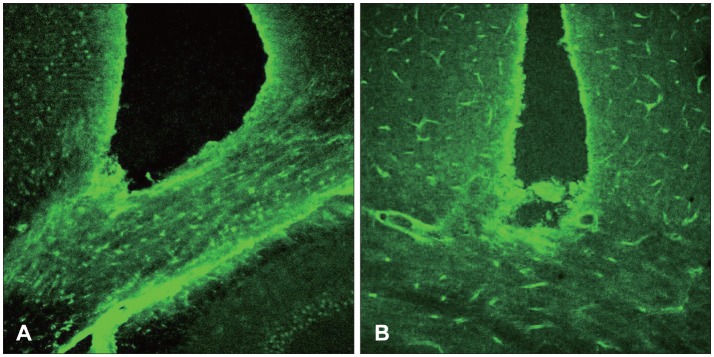
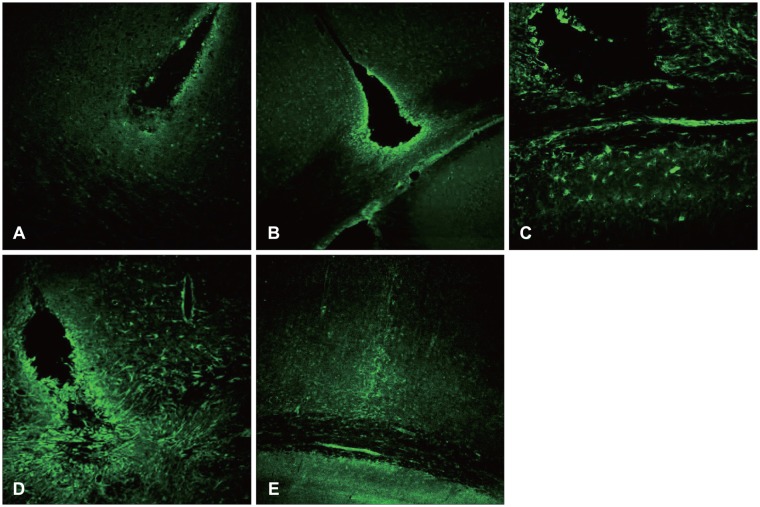
At 12 hours, the GFAP was expressed in the white matter underlying the lesion, and in the cerebral cortex. Nestin was expressed in the astrocytes in the perilesional area after 3 days, while A2B5 was observed in the edge of the wound at 12 hours post-injury, with its expression reaching a peak at 7 days. Vimentin was detected in the white matter at 12 hours, and in the cortex, reaching a peak at 7 days.
CONCLUSION
In the processes of glial scar formation, nestin, vimentin, and A2B5 were revealed in the astrocytes, and these factors may be involved in the division, proliferation, and transportation of the astrocytes.
Keyword
MeSH Terms
Figure
Reference
-
1. Arneman DK, Anton ES. The role of palladin in actin organization: implications for the glial scar. Chapel Hill, NC: University of North Carolina at Chapel Hill;2007.2. Baldwin SA, Scheff SW. Intermediate filament change in astrocytes following mild cortical contusion. Glia. 1996; 16:266–275. PMID: 8833197.
Article3. Calvo JL, Carbonell AL, Boya J. Co-expression of glial fibrillary acidic protein and vimentin in reactive astrocytes following brain injury in rats. Brain Res. 1991; 566:333–336. PMID: 1814551.4. Davies SJ, Goucher DR, Doller C, Silver J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J Neurosci. 1999; 19:5810–5822. PMID: 10407022.
Article5. Douen AG, Dong L, Vanance S, Munger R, Hogan MJ, Thompson CS, et al. Regulation of nestin expression after cortical ablation in adult rat brain. Brain Res. 2004; 1008:139–146. PMID: 15145750.
Article6. Drago J, Reid KL, Bartlett PF. Induction of the ganglioside marker A2B5 on cultured cerebellar neural cells by growth factors. Neurosci Lett. 1989; 107:245–250. PMID: 2616036.
Article7. Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999; 49:377–391. PMID: 10483914.
Article8. Goings GE, Sahni V, Szele FG. Migration patterns of subventricular zone cells in adult mice change after cerebral cortex injury. Brain Res. 2004; 996:213–226. PMID: 14697499.
Article9. Holmin S, Almqvist P, Lendahl U, Mathiesen T. Adult nestin-expressing subependymal cells differentiate to astrocytes in response to brain injury. Eur J Neurosci. 1997; 9:65–75. PMID: 9042570.
Article10. Lee CY, Pappas GD, Kriho V, Huang BM, Yang HY. Proliferation of a subpopulation of reactive astrocytes following needle-insertion lesion in rat. Neurol Res. 2003; 25:767–776. PMID: 14579798.
Article11. Mocchetti I, Wrathall JR. Neurotrophic factors in central nervous system trauma. J Neurotrauma. 1995; 12:853–870. PMID: 8594213.
Article12. Moon C, Ahn M, Kim S, Jin JK, Sim KB, Kim HM, et al. Temporal patterns of the embryonic intermediate filaments nestin and vimentin expression in the cerebral cortex of adult rats after cryoinjury. Brain Res. 2004; 1028:238–242. PMID: 15527750.
Article13. Mothe AJ, Tator CH. Proliferation, migration, and differentiation of endogenous ependymal region stem/progenitor cells following minimal spinal cord injury in the adult rat. Neuroscience. 2005; 131:177–187. PMID: 15680701.
Article14. Nieto-Sampedro M. Neurite outgrowth inhibitors in gliotic tissue. Adv Exp Med Biol. 1999; 468:207–224. PMID: 10635031.
Article15. Ramer MS, Harper GP, Bradbury EJ. Progress in spinal cord research - a refined strategy for the International Spinal Research Trust. Spinal Cord. 2000; 38:449–472. PMID: 10962607.
Article16. Rao MS, Noble M, Mayer-Pröschel M. A tripotential glial precursor cell is present in the developing spinal cord. Proc Natl Acad Sci U S A. 1998; 95:3996–4001. PMID: 9520481.
Article17. Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997; 20:570–577. PMID: 9416670.
Article18. Scolding NJ, Rayner PJ, Compston DA. Identification of A2B5-positive putative oligodendrocyte progenitor cells and A2B5-positive astrocytes in adult human white matter. Neuroscience. 1999; 89:1–4. PMID: 10051212.
Article19. Steeves JD, Tetzlaff W. Engines, accelerators, and brakes on functional spinal cord repair. Ann N Y Acad Sci. 1998; 860:412–424. PMID: 9928328.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regional heterogeneity of morphological changes in cultured rat astrocytes
- Brain-Derived Neurotrophic Factor (BDNF) Exerts a Protective Effect via an Anti-Apoptotic Mechanism on Hypoxic-Ischemic Injury in the Rat Brain
- Astrocytic Expression of CTMP Following an Excitotoxic Lesion in the Mouse Hippocampus
- The Oligomeric Form of Amyloid Beta Triggers Astrocyte Activation, Independent of Neurons
- Late Passage Cultivation Induces Aged Astrocyte Phenotypes in Rat Primary Cultured Cells