Cancer Res Treat.
2017 Apr;49(2):338-349. 10.4143/crt.2016.175.
Adipose Stromal Cells from Visceral and Subcutaneous Fat Facilitate Migration of Ovarian Cancer Cells via IL-6/JAK2/STAT3 Pathway
- Affiliations
-
- 1Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea. yssong@snu.ac.kr
- 2Nano System Institute, Seoul National University, Seoul, Korea.
- 3Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul, Korea.
- 4Departments of Obstetrics & Gynecology and Cellular & Molecular Medicine, Interdisciplinary School of Health Sciences, University of Ottawa, and Chronic Disease Program, Ottawa Hospital Research Institute, Ottawa, ON, Canada.
- 5Peggy and Stephenson Cancer Center, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA.
- 6WCU Biomodulation, Department of Agricultural Biotechnology, Seoul National University, Seoul, Korea.
- KMID: 2378106
- DOI: http://doi.org/10.4143/crt.2016.175
Abstract
- PURPOSE
Adipose stromal cells (ASCs) play an important regulatory role in cancer progression and metastasis by regulating systemic inflammation and tissue metabolism. This study examined whether visceral and subcutaneous ASCs (V- and S-ASCs) facilitate the growth and migration of ovarian cancer cells.
MATERIALS AND METHODS
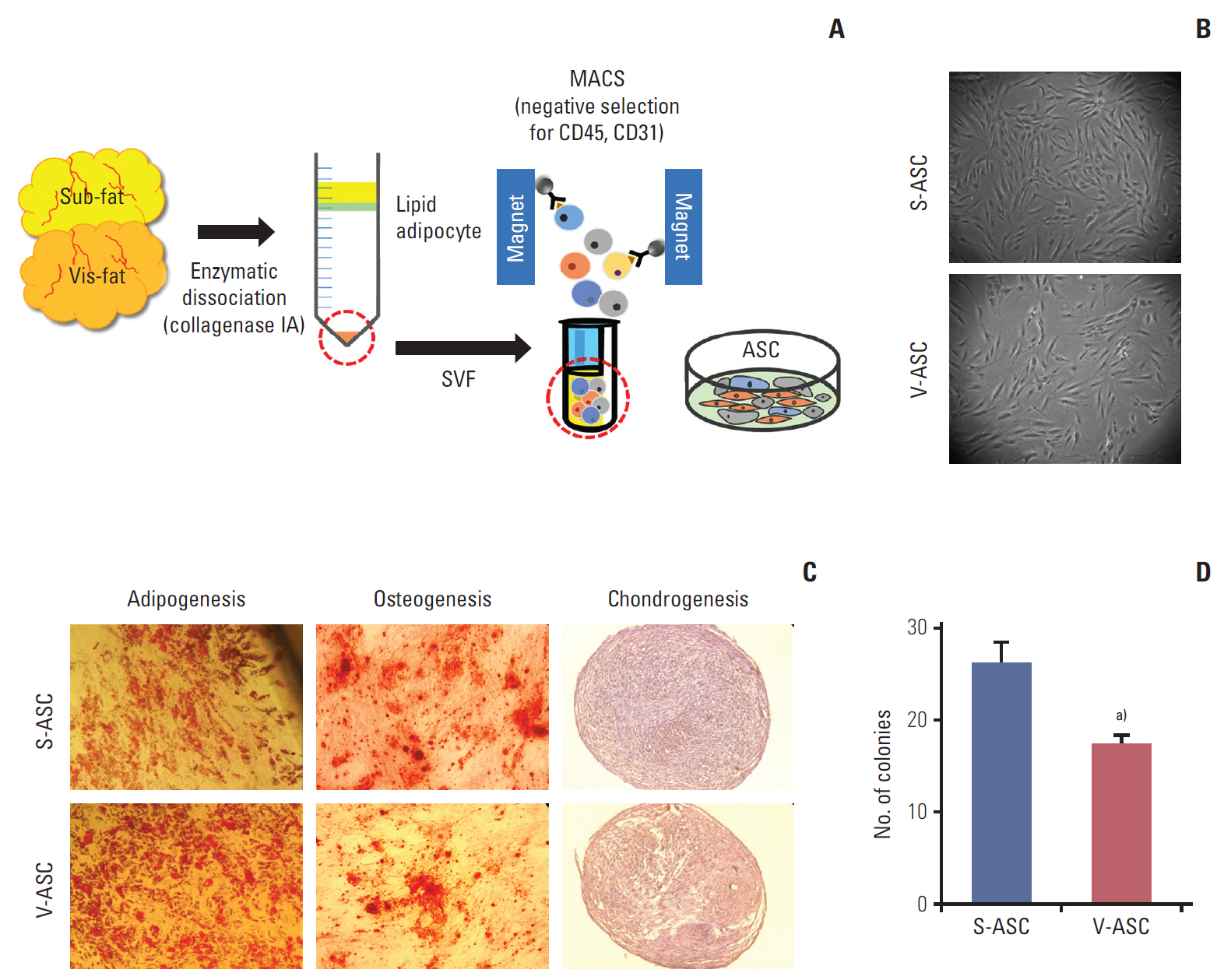
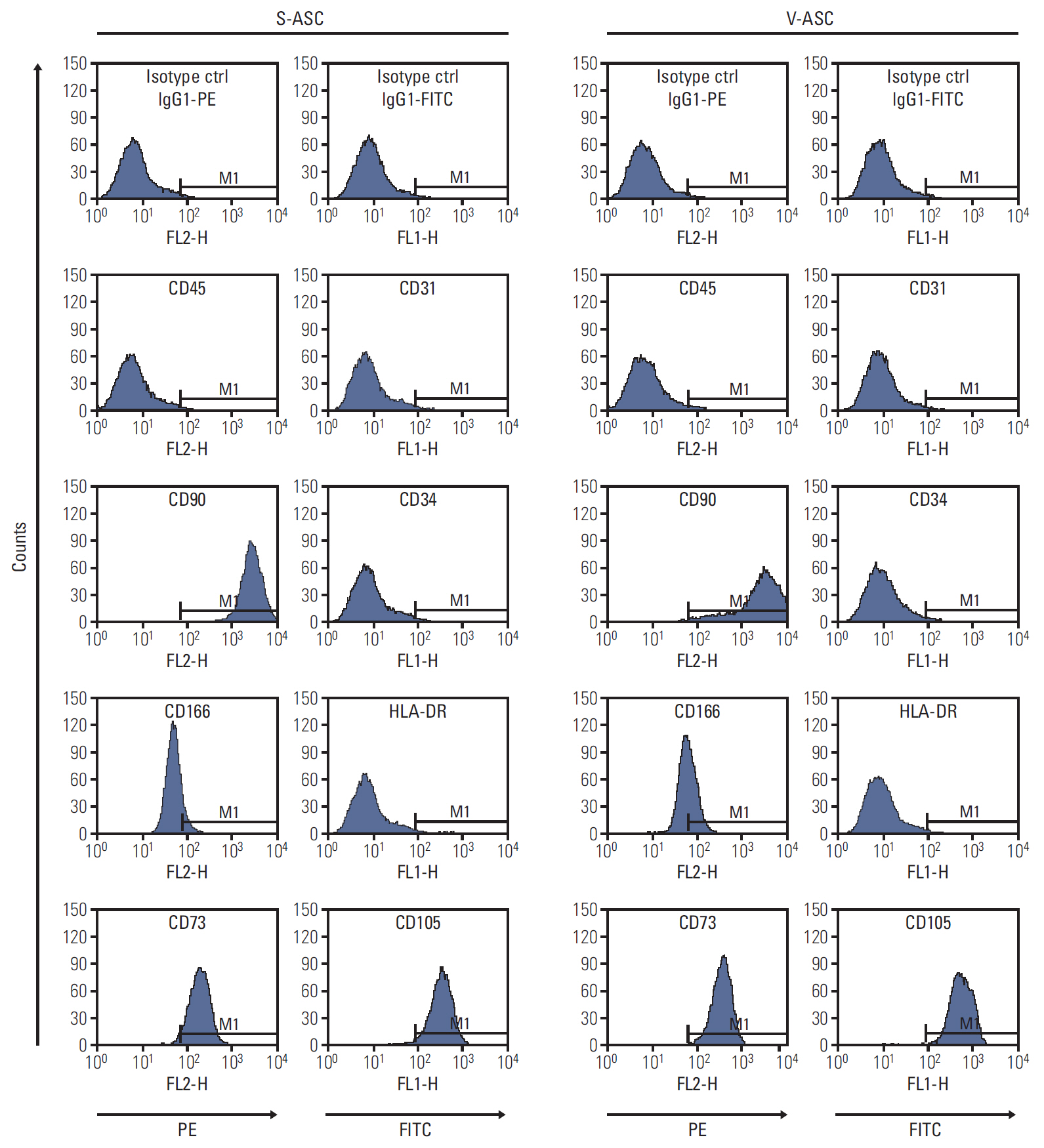
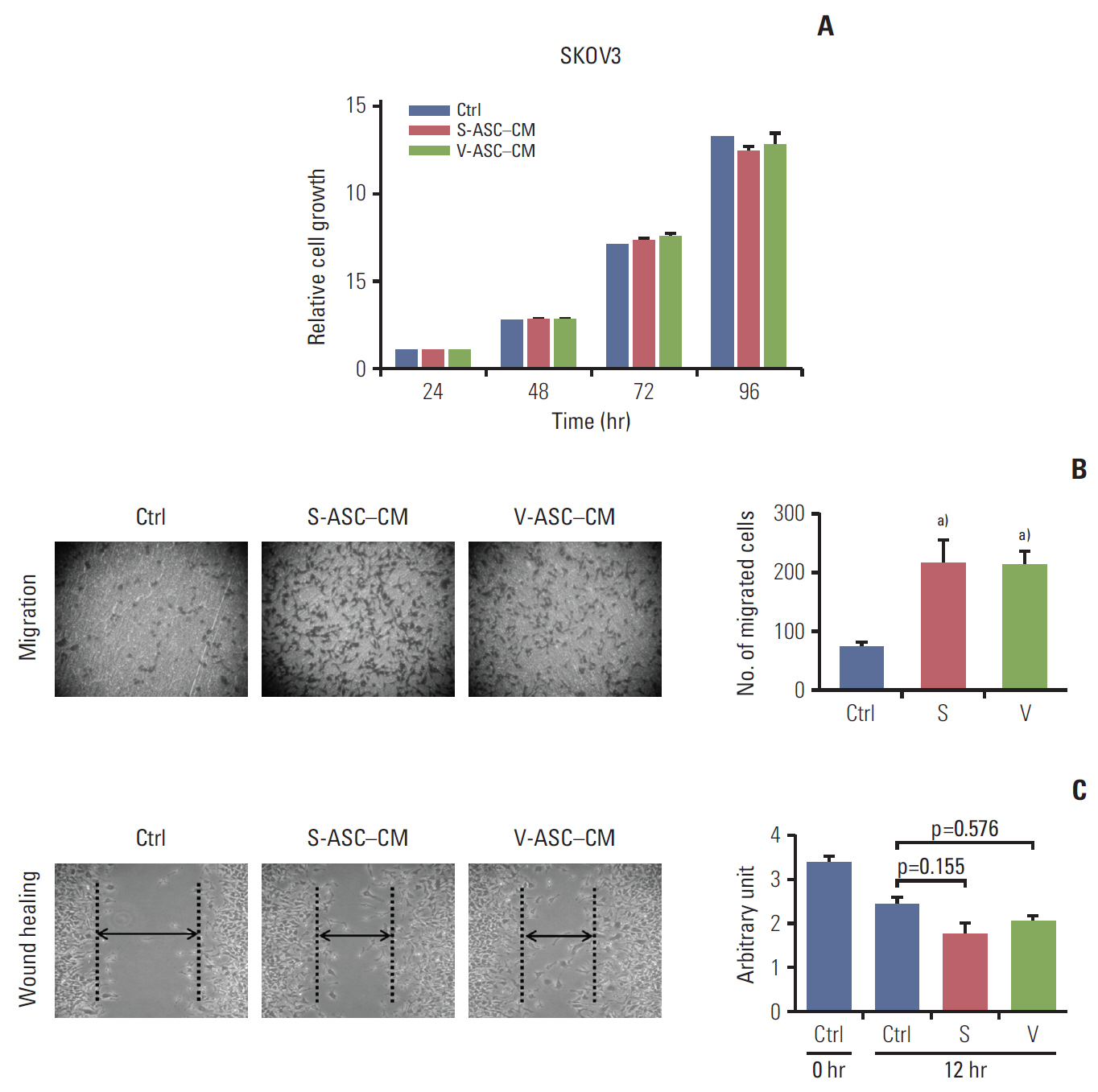
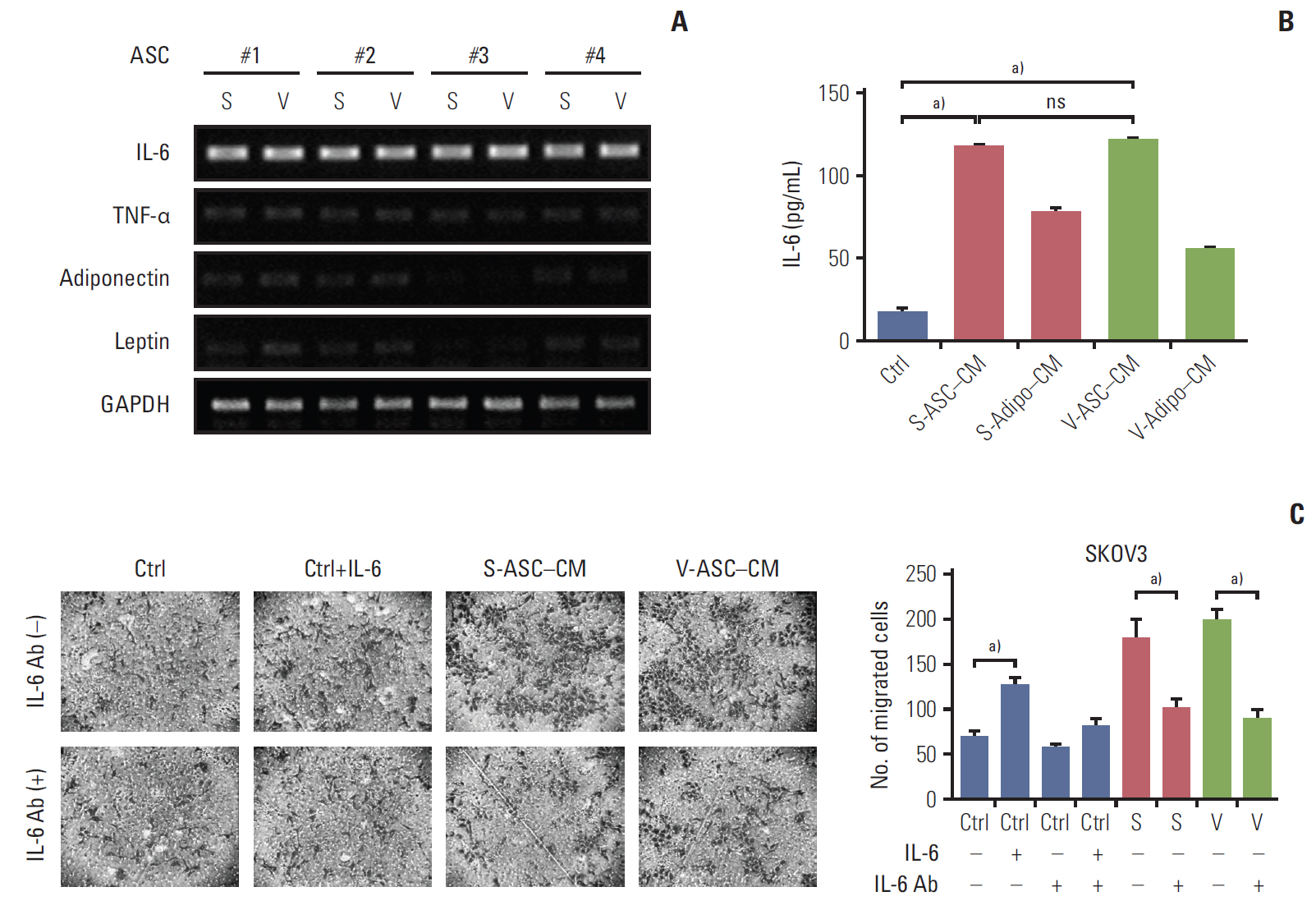
CD45- and CD31- double-negative ASCs were isolated from the subcutaneous and visceral fat using magnetic-activated cell sorting. Ovarian cancer cells were cultured in conditioned media (CM) obtained from ASCs to determine the cancer-promoting effects of ASCs. A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay, Boyden chamber assay, and western blotting were performed to determine the proliferative activity, migration ability, and activation of the JAK2/STAT3 pathway, respectively.
RESULTS
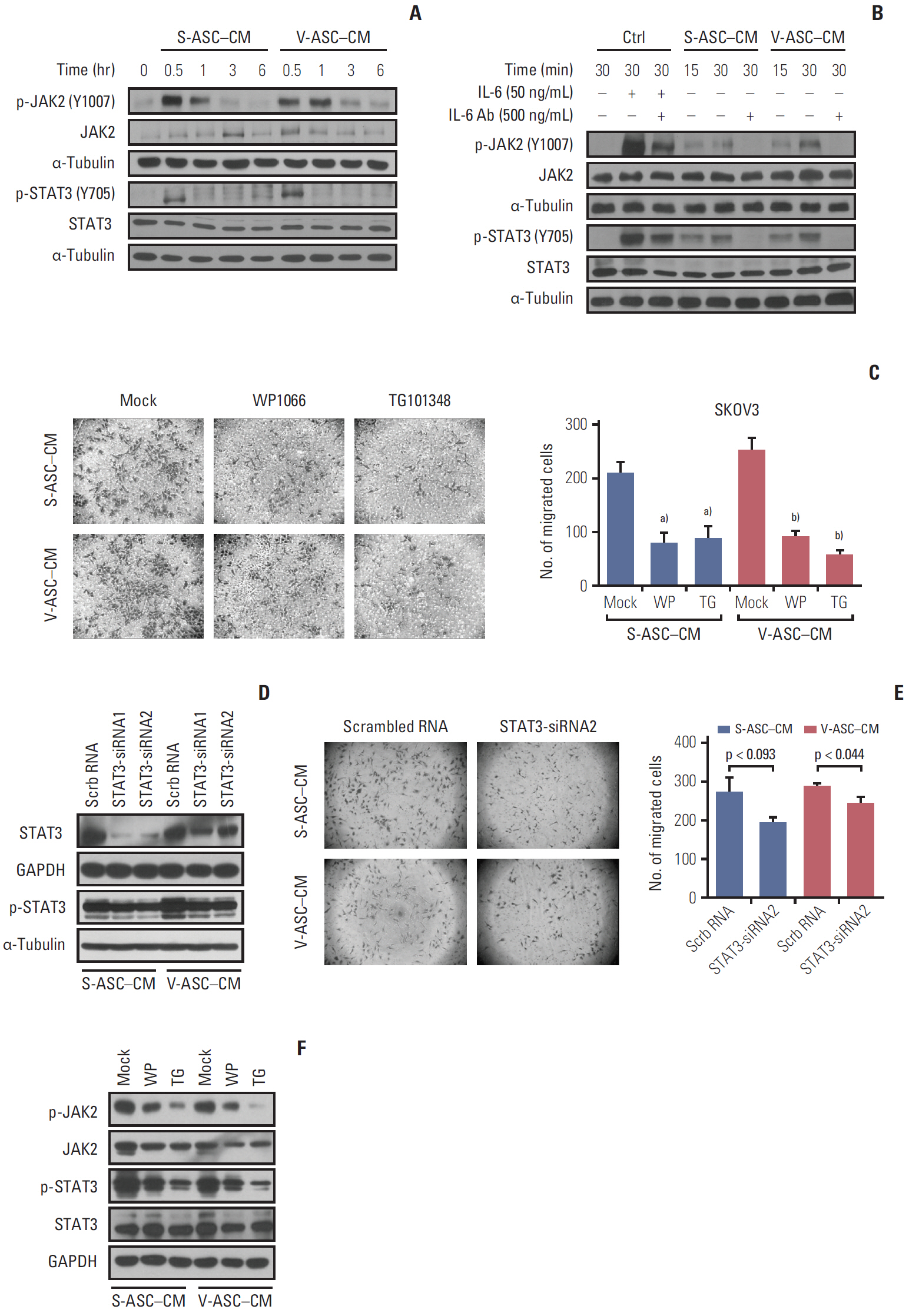
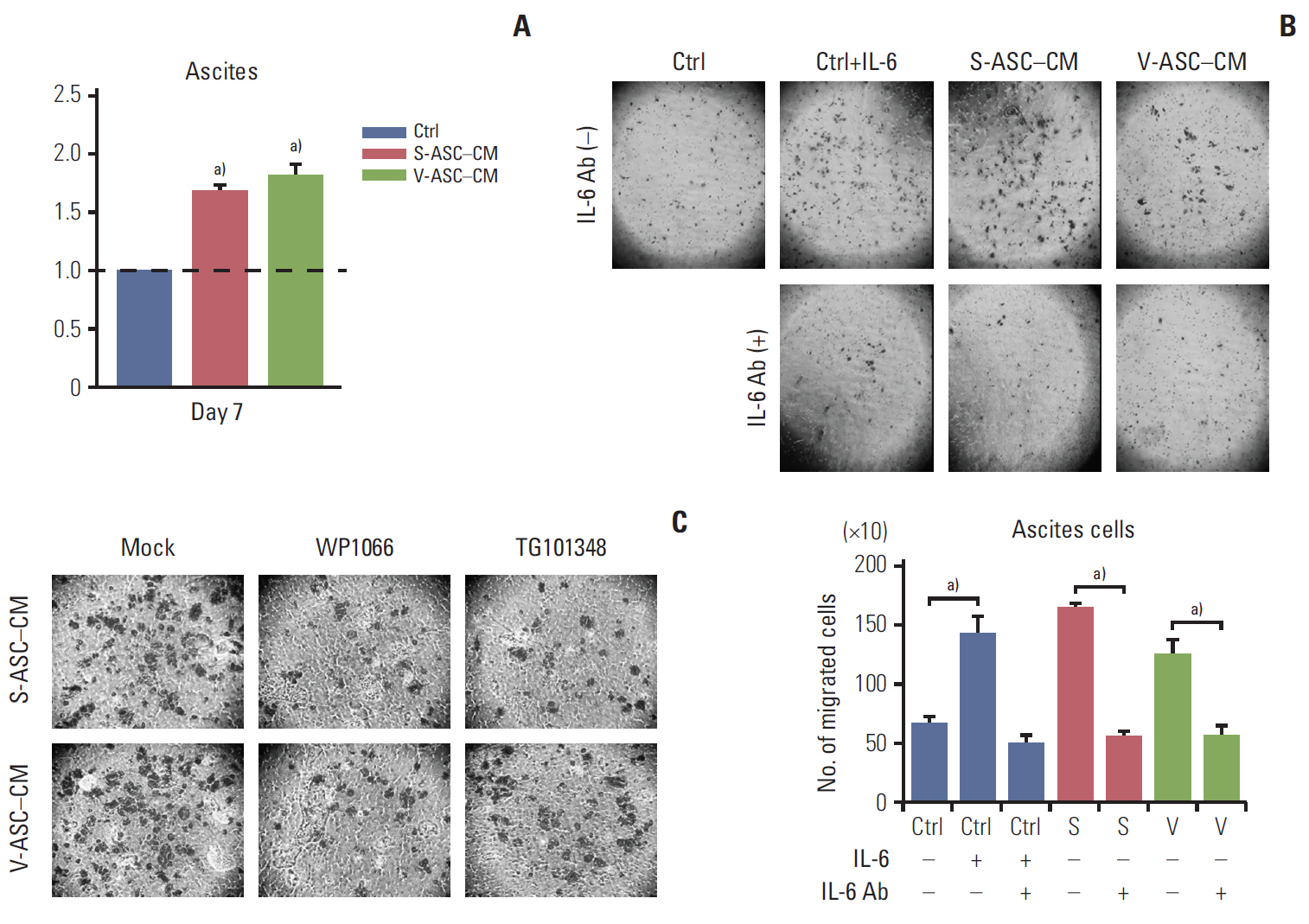
CM from ASCs enhanced the migration of the ovarian cancer line, SKOV3, via activation of the JAK2/STAT3 signaling pathway. Interestingly, in response to ASC-CM, the ascites cells derived from an ovarian cancer patient showed an increase in growth and migration. The migration of ovarian cancer cells was suppressed by blocking the activation of JAK2 and STAT3 using a neutralizing antibody against interleukin 6, small molecular inhibitors (e.g., WP1066 and TG101348), and silencing of STAT3 using siRNA. Anatomical differences between S- and V-ASCs did not affect the growth and migration of the ovarian cancer cell line and ascites cells from the ovarian cancer patients.
CONCLUSION
ASCs may regulate the progression of ovarian cancer, and possibly provide a potential target for anticancer therapy.
MeSH Terms
-
Adipose Tissue
Antibodies, Neutralizing
Ascites
Blotting, Western
Cell Line
Cell Movement
Culture Media, Conditioned
Humans
Inflammation
Interleukin-6
Intra-Abdominal Fat
Metabolism
Neoplasm Metastasis
Ovarian Neoplasms*
RNA, Small Interfering
Stromal Cells*
Subcutaneous Fat*
Antibodies, Neutralizing
Culture Media, Conditioned
Interleukin-6
RNA, Small Interfering
Figure
Reference
-
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
Article2. Lowe KA, Chia VM, Taylor A, O'Malley C, Kelsh M, Mohamed M, et al. An international assessment of ovarian cancer incidence and mortality. Gynecol Oncol. 2013; 130:107–14.
Article3. Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010; 177:1053–64.
Article4. Naora H. Heterotypic cellular interactions in the ovarian tumor microenvironment: biological significance and therapeutic implications. Front Oncol. 2014; 4:18.
Article5. Naora H, Montell DJ. Ovarian cancer metastasis: integrating insights from disparate model organisms. Nat Rev Cancer. 2005; 5:355–66.
Article6. Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011; 17:1498–503.
Article7. Gilbert CA, Slingerland JM. Cytokines, obesity, and cancer: new insights on mechanisms linking obesity to cancer risk and progression. Annu Rev Med. 2013; 64:45–57.
Article8. Trayhurn P, Beattie JH. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc. 2001; 60:329–39.
Article9. Kilroy GE, Foster SJ, Wu X, Ruiz J, Sherwood S, Heifetz A, et al. Cytokine profile of human adipose-derived stem cells: expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol. 2007; 212:702–9.
Article10. Trayhurn P. Endocrine and signalling role of adipose tissue: new perspectives on fat. Acta Physiol Scand. 2005; 184:285–93.
Article11. Wang M, Crisostomo PR, Herring C, Meldrum KK, Meldrum DR. Human progenitor cells from bone marrow or adipose tissue produce VEGF, HGF, and IGF-I in response to TNF by a p38 MAPK-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2006; 291:R880–4.
Article12. Prantl L, Muehlberg F, Navone NM, Song YH, Vykoukal J, Logothetis CJ, et al. Adipose tissue-derived stem cells promote prostate tumor growth. Prostate. 2010; 70:1709–15.
Article13. Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007; 449:557–63.
Article14. Klopp AH, Zhang Y, Solley T, Amaya-Manzanares F, Marini F, Andreeff M, et al. Omental adipose tissue-derived stromal cells promote vascularization and growth of endometrial tumors. Clin Cancer Res. 2012; 18:771–82.
Article15. Bjorntorp P. The regulation of adipose tissue distribution in humans. Int J Obes Relat Metab Disord. 1996; 20:291–302.16. Baglioni S, Cantini G, Poli G, Francalanci M, Squecco R, Di Franco A, et al. Functional differences in visceral and subcutaneous fat pads originate from differences in the adipose stem cell. PLoS One. 2012; 7:e36569.
Article17. Perrini S, Ficarella R, Picardi E, Cignarelli A, Barbaro M, Nigro P, et al. Differences in gene expression and cytokine release profiles highlight the heterogeneity of distinct subsets of adipose tissue-derived stem cells in the subcutaneous and visceral adipose tissue in humans. PLoS One. 2013; 8:e57892.
Article18. Tchkonia T, Lenburg M, Thomou T, Giorgadze N, Frampton G, Pirtskhalava T, et al. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am J Physiol Endocrinol Metab. 2007; 292:E298–307.
Article19. Kipps E, Tan DS, Kaye SB. Meeting the challenge of ascites in ovarian cancer: new avenues for therapy and research. Nat Rev Cancer. 2013; 13:273–82.
Article20. Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010; 11:11–8.
Article21. Coffelt SB, Marini FC, Watson K, Zwezdaryk KJ, Dembinski JL, LaMarca HL, et al. The pro-inflammatory peptide LL-37 promotes ovarian tumor progression through recruitment of multipotent mesenchymal stromal cells. Proc Natl Acad Sci U S A. 2009; 106:3806–11.
Article22. Lis R, Touboul C, Mirshahi P, Ali F, Mathew S, Nolan DJ, et al. Tumor associated mesenchymal stem cells protects ovarian cancer cells from hyperthermia through CXCL12. Int J Cancer. 2011; 128:715–25.
Article23. Walter M, Liang S, Ghosh S, Hornsby PJ, Li R. Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of breast cancer cells. Oncogene. 2009; 28:2745–55.
Article24. Nowicka A, Marini FC, Solley TN, Elizondo PB, Zhang Y, Sharp HJ, et al. Human omental-derived adipose stem cells increase ovarian cancer proliferation, migration, and chemoresistance. PLoS One. 2013; 8:e81859.
Article25. Domcke S, Sinha R, Levine DA, Sander C, Schultz N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat Commun. 2013; 4:2126.
Article26. Colomiere M, Ward AC, Riley C, Trenerry MK, Cameron-Smith D, Findlay J, et al. Cross talk of signals between EGFR and IL-6R through JAK2/STAT3 mediate epithelial-mesenchymal transition in ovarian carcinomas. Br J Cancer. 2009; 100:134–44.
Article27. Lee JY, Yoon JK, Kim B, Kim S, Kim MA, Lim H, et al. Tumor evolution and intratumor heterogeneity of an epithelial ovarian cancer investigated using next-generation sequencing. BMC Cancer. 2015; 15:85.
Article28. Song H, Sondak VK, Barber DL, Reid TJ, Lin J. Modulation of Janus kinase 2 by cisplatin in cancer cells. Int J Oncol. 2004; 24:1017–26.
Article29. Kim HS, Choi HY, Lee M, Suh DH, Kim K, No JH, et al. Systemic inflammatory response markers and CA-125 levels in ovarian clear cell carcinoma: a two center cohort study. Cancer Res Treat. 2016; 48:250–8.
Article30. Lakshmanan I, Ponnusamy MP, Das S, Chakraborty S, Haridas D, Mukhopadhyay P, et al. MUC16 induced rapid G2/M transition via interactions with JAK2 for increased proliferation and anti-apoptosis in breast cancer cells. Oncogene. 2012; 31:805–17.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Peroxisome Proliferator-activated Receptor-gamma Inhibits the Activation of STAT3 in Cerulein-stimulated Pancreatic Acinar Cells
- RAR-Related Orphan Receptor: An Accelerated Preeclampsia Progression by Activating the JAK/STAT3 Pathway
- Hepatogenic differentiation of human mesenchymal stem cells from peritoneal adipose tissue
- microRNA-146a Promotes Growth of Acute Leukemia Cells by Downregulating Ciliary Neurotrophic Factor Receptor and Activating JAK2/STAT3 Signaling
- Adipose Tissue Derived Mesenchymal Stem Cells