Korean J Pain.
2017 Apr;30(2):86-92. 10.3344/kjp.2017.30.2.86.
Can denosumab be a substitute, competitor, or complement to bisphosphonates?
- Affiliations
-
- 1Department of Anesthesia and Pain Medicine, School of Medicine, Pusan National University, Yangsan, Korea. pain@pusan.ac.kr
- 2Department of Orthopedics, Ludwig-Maximilian-University Munich, Grosshadern Campus, Munich, Germany.
- KMID: 2376043
- DOI: http://doi.org/10.3344/kjp.2017.30.2.86
Abstract
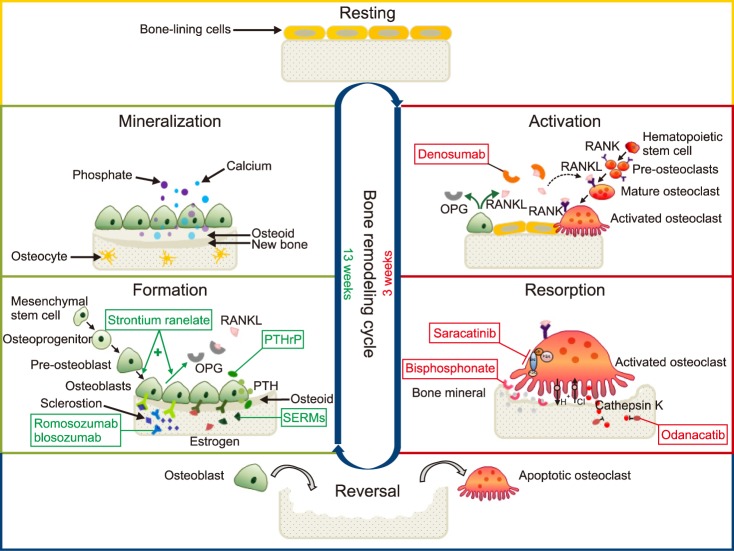
- Osteoblasts, originating from mesenchymal cells, make the receptor activator of the nuclear factor kappa B ligand (RANKL) and osteoprotegerin (OPG) in order to control differentiation of activated osteoclasts, originating from hematopoietic stem cells. When the RANKL binds to the RANK of the pre-osteoclasts or mature osteoclasts, bone resorption increases. On the contrary, when OPG binds to the RANK, bone resorption decreases. Denosumab (AMG 162), like OPG (a decoy receptor), binds to the RANKL, and reduces binding between the RANK and the RANKL resulting in inhibition of osteoclastogenesis and reduction of bone resorption. Bisphosphonates (BPs), which bind to the bone mineral and occupy the site of resorption performed by activated osteoclasts, are still the drugs of choice to prevent and treat osteoporosis. The merits of denosumab are reversibility targeting the RANKL, lack of adverse gastrointestinal events, improved adherence due to convenient biannual subcutaneous administration, and potential use with impaired renal function. The known adverse reactions are musculoskeletal pain, increased infections with adverse dermatologic reactions, osteonecrosis of the jaw, hypersensitivity reaction, and hypocalcemia. Treatment with 60 mg of denosumab reduces the bone resorption marker, serum type 1 C-telopeptide, by 3 days, with maximum reduction occurring by 1 month. The mean time to maximum denosumab concentration is 10 days with a mean half-life of 25.4 days. In conclusion, the convenient biannual subcutaneous administration of 60 mg of denosumab can be considered as a first-line treatment for osteoporosis in cases of low compliance with BPs due to gastrointestinal trouble and impaired renal function.
Keyword
MeSH Terms
-
Antibodies, Monoclonal
Biomarkers
Bone Density
Bone Resorption
Compliance
Denosumab*
Diphosphonates*
Half-Life
Hematopoietic Stem Cells
Hypersensitivity
Hypocalcemia
Jaw
Miners
Musculoskeletal Pain
NF-kappa B
Osteoblasts
Osteoclasts
Osteonecrosis
Osteoporosis
Osteoprotegerin
RANK Ligand
Antibodies, Monoclonal
Biomarkers
Denosumab
Diphosphonates
NF-kappa B
Osteoprotegerin
RANK Ligand
Figure
Cited by 1 articles
-
Medications for osteoporotic pain
Sang Wook Shin
Korean J Pain. 2017;30(2):85-85. doi: 10.3344/kjp.2017.30.2.85.
Reference
-
1. Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014; 25:2359–2381. PMID: 25182228.
Article2. Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008; 83:1032–1045. PMID: 18775204.
Article3. Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone. 2011; 48:677–692. PMID: 21145999.
Article4. McClung MR, Lewiecki EM, Cohen SB, Bolognese MA, Woodson GC, Moffett AH, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006; 354:821–831. PMID: 16495394.
Article5. Cheung AM, Frame H, Ho M, Mackinnon ES, Brown JP. Bone strength and management of postmenopausal fracture risk with antiresorptive therapies: considerations for women's health practice. Int J Womens Health. 2016; 8:537–547. PMID: 27729815.6. Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009; 361:756–765. PMID: 19671655.
Article7. Khosla S. Increasing options for the treatment of osteoporosis. N Engl J Med. 2009; 361:818–820. PMID: 19671654.
Article8. Rizzoli R, Yasothan U, Kirkpatrick P. Denosumab. Nat Rev Drug Discov. 2010; 9:591–592. PMID: 20671758.
Article9. Zaheer S, LeBoff M, Lewiecki EM. Denosumab for the treatment of osteoporosis. Expert Opin Drug Metab Toxicol. 2015; 11:461–470. PMID: 25614274.
Article10. Janjan N. Bone metastases: approaches to management. Semin Oncol. 2001; 28:28–34.
Article11. Weinfurt KP, Li Y, Castel LD, Saad F, Timbie JW, Glendenning GA, et al. The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann Oncol. 2005; 16:579–584. PMID: 15734776.
Article12. Yong M, Jensen AÖ, Jacobsen JB, Nørgaard M, Fryzek JP, Sørensen HT. Survival in breast cancer patients with bone metastases and skeletal-related events: a population-based cohort study in Denmark (1999-2007). Breast Cancer Res Treat. 2011; 129:495–503. PMID: 21461730.
Article13. Cassinello Espinosa J, González Del Alba Baamonde A, Rivera Herrero F, Holgado Martín E. SEOM (Spanish Society of Clinical Oncology). SEOM guidelines for the treatment of bone metastases from solid tumours. Clin Transl Oncol. 2012; 14:505–511. PMID: 22721794.
Article14. von Moos R, Costa L, Ripamonti CI, Niepel D, Santini D. Improving quality of life in patients with advanced cancer: targeting metastatic bone pain. Eur J Cancer. 2017; 71:80–94. PMID: 27984770.
Article15. Raggatt LJ, Partridge NC. Cellular and molecular mechanisms of bone remodeling. J Biol Chem. 2010; 285:25103–25108. PMID: 20501658.
Article16. Proff P, Römer P. The molecular mechanism behind bone remodelling: a review. Clin Oral Investig. 2009; 13:355–362.
Article17. Ghayor C, Weber FE. Epigenetic regulation of bone remodeling and its impacts in osteoporosis. Int J Mol Sci. 2016; 17:E1446. PMID: 27598138.
Article18. Crockett JC, Rogers MJ, Coxon FP, Hocking LJ, Helfrich MH. Bone remodelling at a glance. J Cell Sci. 2011; 124:991–998. PMID: 21402872.
Article19. Miller SC, de Saint-Georges L, Bowman BM, Jee WS. Bone lining cells: structure and function. Scanning Microsc. 1989; 3:953–960. PMID: 2694361.20. Hanley DA, Adachi JD, Bell A, Brown V. Denosumab: mechanism of action and clinical outcomes. Int J Clin Pract. 2012; 66:1139–1146. PMID: 22967310.
Article21. Xing L, Xiu Y, Boyce BF. Osteoclast fusion and regulation by RANKL-dependent and independent factors. World J Orthop. 2012; 3:212–222. PMID: 23362465.
Article22. Martin TJ. Paracrine regulation of osteoclast formation and activity: milestones in discovery. J Musculoskelet Neuronal Interact. 2004; 4:243–253. PMID: 15615492.23. Clarke BL. Anti-sclerostin antibodies: utility in treatment of osteoporosis. Maturitas. 2014; 78:199–204. PMID: 24842796.
Article24. Boivin G, Farlay D, Bala Y, Doublier A, Meunier PJ, Delmas PD. Influence of remodeling on the mineralization of bone tissue. Osteoporos Int. 2009; 20:1023–1026. PMID: 19340504.
Article25. Bone HG, Bolognese MA, Yuen CK, Kendler DL, Wang H, Liu Y, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women. J Clin Endocrinol Metab. 2008; 93:2149–2157. PMID: 18381571.
Article26. Sohn W, Simiens MA, Jaeger K, Hutton S, Jang G. The pharmacokinetics and pharmacodynamics of denosumab in patients with advanced solid tumours and bone metastases: a systematic review. Br J Clin Pharmacol. 2014; 78:477–487. PMID: 24548274.
Article27. Miller PD. A review of the efficacy and safety of denosumab in postmenopausal women with osteoporosis. Ther Adv Musculoskelet Dis. 2011; 3:271–282. PMID: 22870485.
Article28. Martin M, Bell R, Bourgeois H, Brufsky A, Diel I, Eniu A, et al. Bone-related complications and quality of life in advanced breast cancer: results from a randomized phase III trial of denosumab versus zoledronic acid. Clin Cancer Res. 2012; 18:4841–4849. PMID: 22893628.
Article29. Kendler DL, Roux C, Benhamou CL, Brown JP, Lillestol M, Siddhanti S, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res. 2010; 25:72–81. PMID: 19594293.
Article30. Fizazi K, Carducci M, Smith M, Damião R, Brown J, Karsh L, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011; 377:813–822. PMID: 21353695.
Article31. Anastasilakis AD, Polyzos SA, Anastasilakis CD, Toulis KA, Makras P. Denosumab and bisphosphonates: rivals or potential “partners”? A “hybrid” molecule hypothesis. Med Hypotheses. 2011; 77:109–111. PMID: 21482033.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Bisphosphonates Prior to Denosumab Treatment on Rebound Fractures: A Mini Review
- Review on the comparison of effectiveness between denosumab and bisphosphonates in post-menopausal osteoporosis
- Bone-modifying agents for bone metastasis in patients with breast cancer
- Medication Use Evaluation of Denosumab in Postmenopausal Women with Osteoporosis or Osteopenia
- Comparison of the Effectiveness and Hypocalcemia Risk of Antiresorptive Agents in Patients with Hypercalcemia of Malignancy