Yonsei Med J.
2016 May;57(3):647-651. 10.3349/ymj.2016.57.3.647.
Activation of NF-κB and AP-1 Mediates Hyperproliferation by Inducing β-Catenin and c-Myc in Helicobacter pylori-Infected Gastric Epithelial Cells
- Affiliations
-
- 1Department of Food and Nutrition, Brain Korea 21 PLUS Project, College of Human Ecology, Yonsei University, Seoul, Korea. kim626@yonsei.ac.kr
- 2Department of Pharmacology, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2374084
- DOI: http://doi.org/10.3349/ymj.2016.57.3.647
Abstract
- PURPOSE
In the gastric mucosa of Helicobacter pylori (H. pylori)-infected patients with gastritis or adenocarcinoma, proliferation of gastric epithelial cells is increased. Hyperproliferation is related to induction of oncogenes, such as β-catenin and c-myc. Even though transcription factors NF-κB and AP-1 are activated in H. pylori-infected cells, whether NF-κB or AP-1 regulates the expression of β-catenein or c-myc in H. pylori-infected cells has not been clarified. The present study was undertaken to investigate whether H. pylori-induced activation of NF-κB and AP-1 mediates the expression of oncogenes and hyperproliferation of gastric epithelial cells.
MATERIALS AND METHODS
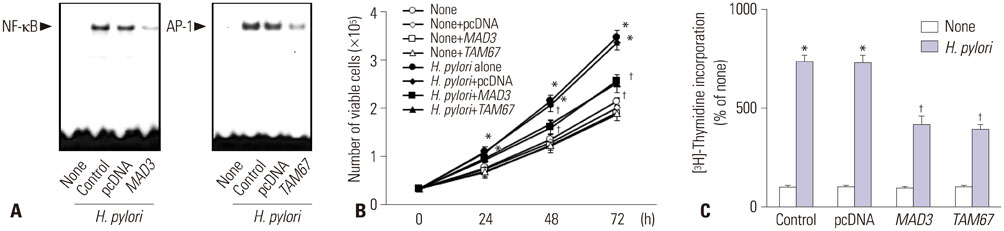
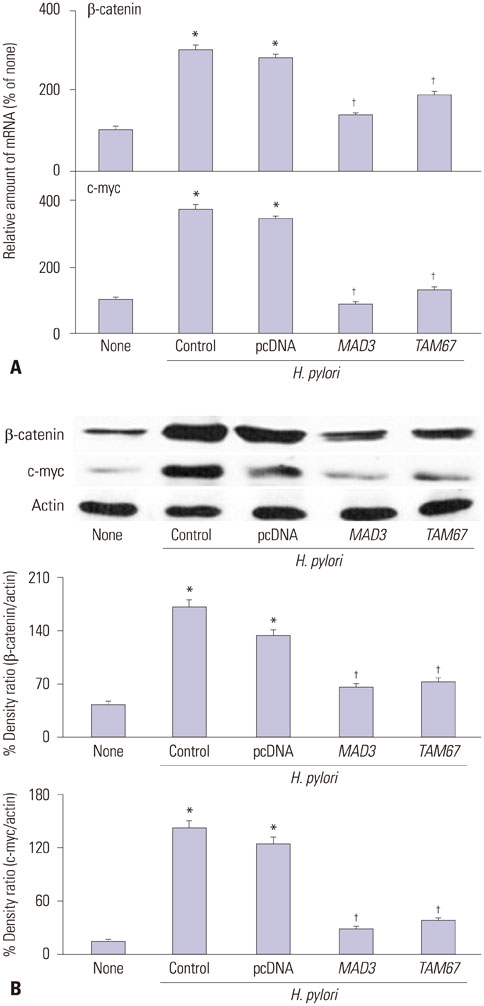
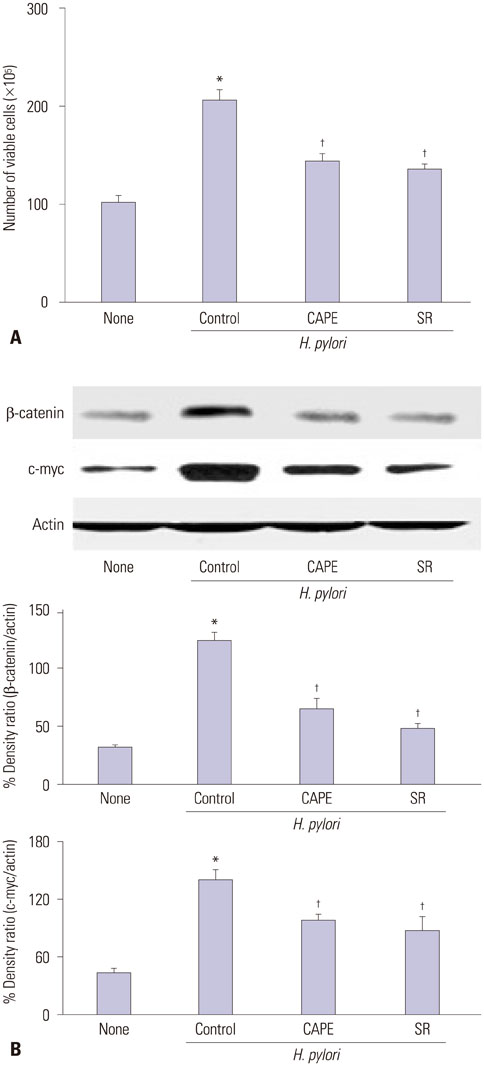
Gastric epithelial AGS cells were transiently transfected with mutant genes for IκBα (MAD3) and c-Jun (TAM67) or treated with a specific NF-κB inhibitor caffeic acid phenethyl ester (CAPE) or a selective AP-1 inhibitor SR-11302 to suppress activation of NF-κB or AP-1, respecively. As reference cells, the control vector pcDNA was transfected to the cells. Wild-type cells or transfected cells were cultured with or without H. pylori.
RESULTS
H. pylori induced activation of NF-κB and AP-1, cell proliferation, and expression of oncogenes (β-catenein, c-myc) in AGS cells, which was inhibited by transfection of MAD3 and TAM67. Wild-type cells and the cells transfected with pcDNA showed similar activities of NF-κB and AP-1, proliferation, and oncogene expression regardless of treatment with H. pylori. Both CAPE and SR-11302 inhibited cell proliferation and expression of oncogenes in H. pylori-infected cells.
CONCLUSION
H. pylori-induced activation of NF-κB and AP-1 regulates transcription of oncogenes and mediates hyperproliferation in gastric epithelial cells.
Keyword
MeSH Terms
-
Blotting, Western
Caffeic Acids
Cell Line, Tumor
Cell Proliferation
DNA, Bacterial/analysis/genetics
DNA-Binding Proteins/*metabolism
Epithelial Cells/*metabolism
Gastric Mucosa/*metabolism/pathology
Gastritis/pathology
Gene Expression Regulation, Bacterial
Helicobacter Infections/metabolism/pathology/physiopathology
Helicobacter pylori/pathogenicity/physiology
Humans
NF-kappa B/antagonists & inhibitors/*biosynthesis/metabolism
Peptide Fragments
Phenylethyl Alcohol/analogs & derivatives
Proto-Oncogene Proteins c-jun
Repressor Proteins
Transcription Factor AP-1/*biosynthesis
Transcription Factors/*metabolism
beta Catenin/*metabolism
Caffeic Acids
DNA, Bacterial
DNA-Binding Proteins
NF-kappa B
Peptide Fragments
Phenylethyl Alcohol
Proto-Oncogene Proteins c-jun
Repressor Proteins
Transcription Factor AP-1
Transcription Factors
beta Catenin
Figure
Reference
-
1. Negrini R, Savio A, Poiesi C, Appelmelk BJ, Buffoli F, Paterlini A, et al. Antigenic mimicry between Helicobacter pylori and gastric mucosa in the pathogenesis of body atrophic gastritis. Gastroenterology. 1996; 111:655–665.
Article2. Nagy TA, Wroblewski LE, Wang D, Piazuelo MB, Delgado A, Romero-Gallo J, et al. β-Catenin and p120 mediate PPARδ-dependent proliferation induced by Helicobacter pylori in human and rodent epithelia. Gastroenterology. 2011; 141:553–564.
Article3. Peek RM Jr, Moss SF, Tham KT, Pérez-Pérez GI, Wang S, Miller GG, et al. Helicobacter pylori cagA+strains and dissociation of gastric epithelial cell proliferation from apoptosis. J Natl Cancer Inst. 1997; 89:863–868.
Article4. Gnad T, Feoktistova M, Leverkus M, Lendeckel U, Naumann M. Helicobacter pylori-induced activation of beta-catenin involves low density lipoprotein receptor-related protein 6 and Dishevelled. Mol Cancer. 2010; 9:31.5. Behrens J, Jerchow BA, Würtele M, Grimm J, Asbrand C, Wirtz R, et al. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998; 280:596–599.
Article6. Behrens J, von Kries JP, Kühl M, Bruhn L, Wedlich D, Grosschedl R, et al. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996; 382:638–642.
Article7. Shih IM, Yu J, He TC, Vogelstein B, Kinzler KW. The beta-catenin binding domain of adenomatous polyposis coli is sufficient for tumor suppression. Cancer Res. 2000; 60:1671–1676.8. Dang CV. c-Myc target genes involved in cell growth, apoptosis, and metabolism. Mol Cell Biol. 1999; 19:1–11.
Article9. Shimoyama T, Fukuda S, Liu Q, Nakaji S, Fukuda Y, Sugawara K. Production of chemokines and reactive oxygen species by human neutrophils stimulated by Helicobacter pylori. Helicobacter. 2002; 7:170–174.
Article10. Davies GR, Simmonds NJ, Stevens TR, Sheaff MT, Banatvala N, Laurenson IF, et al. Helicobacter pylori stimulates antral mucosal reactive oxygen metabolite production in vivo. Gut. 1994; 35:179–185.
Article11. Byun E, Lim JW, Kim JM, Kim H. α-Lipoic acid inhibits Helicobacter pylori-induced oncogene expression and hyperproliferation by suppressing the activation of NADPH oxidase in gastric epithelial cells. Mediators Inflamm. 2014; 2014:380830.12. Keenan JI, Peterson RA 2nd, Hampton MB. NADPH oxidase involvement in the pathology of Helicobacter pylori infection. Free Radic Biol Med. 2005; 38:1188–1196.13. Tominaga K, Kawahara T, Sano T, Toida K, Kuwano Y, Sasaki H, et al. Evidence for cancer-associated expression of NADPH oxidase 1 (Nox1)-based oxidase system in the human stomach. Free Radic Biol Med. 2007; 43:1627–1638.
Article14. Seo JH, Lim JW, Kim H, Kim KH. Helicobacter pylori in a Korean isolate activates mitogen-activated protein kinases, AP-1, and NF-kappaB and induces chemokine expression in gastric epithelial AGS cells. Lab Invest. 2004; 84:49–62.
Article15. Müller JM, Rupec RA, Baeuerle PA. Study of gene regulation by NF-kappa B and AP-1 in response to reactive oxygen intermediates. Methods. 1997; 11:301–312.
Article16. Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002; 109:S81–S96.17. Chu SH, Kim H, Seo JY, Lim JW, Mukaida N, Kim KH. Role of NF-kappaB and AP-1 on Helicobater pylori-induced IL-8 expression in AGS cells. Dig Dis Sci. 2003; 48:257–265.18. Nollet F, Berx G, Molemans F, van Roy F. Genomic organization of the human beta-catenin gene (CTNNB1). Genomics. 1996; 32:413–424.
Article19. Lim JW, Kim H, Kim KH. NF-kappaB, inducible nitric oxide synthase and apoptosis by Helicobacter pylori infection. Free Radic Biol Med. 2001; 31:355–366.
Article20. Coward L, Smith M, Kirk M, Barnes S. Chemical modification of isoflavones in soyfoods during cooking and processing. Am J Clin Nutr. 1998; 68:6 Suppl. 1486S–1491S.
Article21. Chen MF, Keng PC, Lin PY, Yang CT, Liao SK, Chen WC. Caffeic acid phenethyl ester decreases acute pneumonitis after irradiation in vitro and in vivo. BMC Cancer. 2005; 5:158.
Article22. Jung WK, Choi I, Lee DY, Yea SS, Choi YH, Kim MM, et al. Caffeic acid phenethyl ester protects mice from lethal endotoxin shock and inhibits lipopolysaccharide-induced cyclooxygenase-2 and inducible nitric oxide synthase expression in RAW 264.7 macrophages via the p38/ERK and NF-kappaB pathways. Int J Biochem Cell Biol. 2008; 40:2572–2582.
Article23. Fanjul A, Dawson MI, Hobbs PD, Jong L, Cameron JF, Harlev E, et al. A new class of retinoids with selective inhibition of AP-1 inhibits proliferation. Nature. 1994; 372:107–111.
Article24. Huang C, Ma WY, Dawson MI, Rincon M, Flavell RA, Dong Z. Blocking activator protein-1 activity, but not activating retinoic acid response element, is required for the antitumor promotion effect of retinoic acid. Proc Natl Acad Sci U S A. 1997; 94:5826–5830.
Article25. Bandapalli OR, Dihlmann S, Helwa R, Macher-Goeppinger S, Weitz J, Schirmacher P, et al. Transcriptional activation of the beta-catenin gene at the invasion front of colorectal liver metastases. J Pathol. 2009; 218:370–379.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- β-carotene Inhibits Expression of c-Myc and Cyclin E in Helicobacter pylori-infected Gastric Epithelial Cells
- Caspase-3 Activation Leads to Apoptosis of Human Gastric Epithelial Cells Infected with Helicobacter pylori
- The effects of ecabet sodium on nuclear factor-kappa B activation and chemokine gene expression in Helicobacter pylori-infected human gastric epithelial cells
- Jak1/Stat3 Is an Upstream Signaling of NF-kappaB Activation in Helicobacter pylori-Induced IL-8 Production in Gastric Epithelial AGS Cells
- Steamed Ginger Extract Exerts Anti-inflammatory Effects in Helicobacter pylori-infected Gastric Epithelial Cells through Inhibition of NF-κB