Yonsei Med J.
2016 May;57(3):588-598. 10.3349/ymj.2016.57.3.588.
Tolfenamic Acid Inhibits the Proliferation, Migration, and Invasion of Nasopharyngeal Carcinoma: Involvement of p38-Mediated Down-Regulation of Slug
- Affiliations
-
- 1Department of Otolaryngology, School of Medicine, Ajou University, Suwon, Korea. ostium@ajou.ac.kr
- 2Center of Excellent in Otorhinolaryngology, Head and Neck Surgery, Rajavithi Hospital, Bangkok, Thailand. drpackdee@gmail.com
- 3Department of Molecular Science and Technology, Ajou University, Suwon, Korea.
- KMID: 2374076
- DOI: http://doi.org/10.3349/ymj.2016.57.3.588
Abstract
- PURPOSE
Tolfenamic acid (TA), a non-steroidal anti-inflammatory drug, is known to exhibit antitumor effects in various cancers apart from nasopharyngeal cancer (NPC). NPC exhibits high invasiveness, as well as metastatic potential, and patients continue to suffer from residual, recurrent, or metastatic disease even after chemoradiation therapy. Therefore, new treatment strategies are needed for NPC. In this study, we investigated the efficacy and molecular mechanisms of TA in NPC treatment.
MATERIALS AND METHODS
TA-induced cell death was detected by cell viability assay in the NPC cell lines, HNE1 and HONE1. Wound healing assay, invasion assay, and Western blot analysis were used to evaluate the antitumor effects of TA in NPC cell lines.
RESULTS
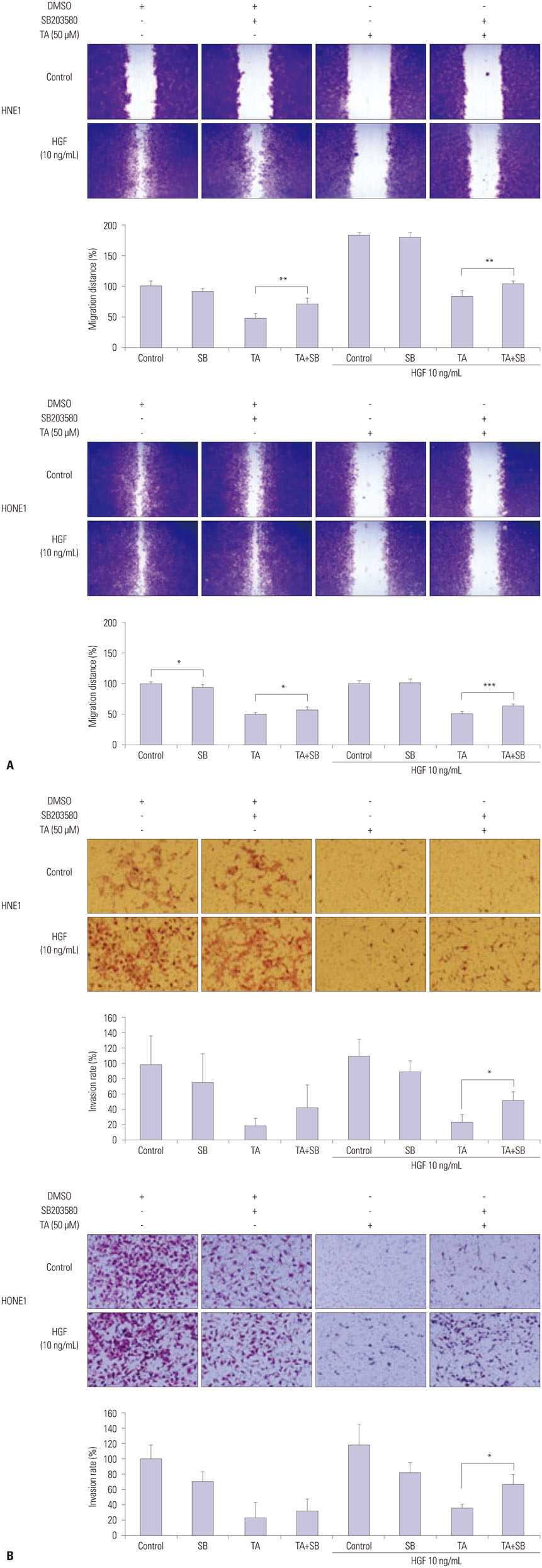
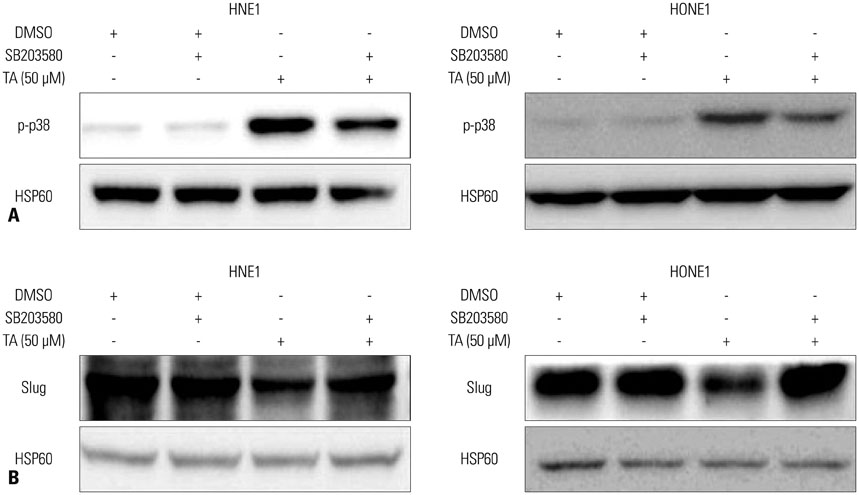
Treatment with TA suppressed the migration and invasion of HNE1 and HONE1 cells. Hepatocyte growth factor enhanced the proliferation, migration, and invasion abilities of NPC cells. This enhancement was successfully inhibited by TA treatment. Treatment with TA increased phosphorylation of p38, and the inhibition of p38 with SB203580 reversed the cytotoxic, anti-invasive, and anti-migratory effects of TA treatment in NPC cell lines. Moreover, inhibition of p38 also reversed the decrease in expression of Slug that was induced by TA treatment.
CONCLUSION
In conclusion, the activation of p38 plays a role in mediating TA-induced cytotoxicity and inhibition of invasion and migration via down-regulation of Slug.
MeSH Terms
-
Animals
Anti-Inflammatory Agents, Non-Steroidal/*pharmacology/therapeutic use
Cell Line, Tumor
Cell Movement/*drug effects
Cell Proliferation/*drug effects
Cell Survival/*drug effects
Down-Regulation
Gastropoda
Gene Expression Regulation, Neoplastic/drug effects
Hepatocyte Growth Factor/metabolism/*pharmacology
Humans
Imidazoles
MAP Kinase Signaling System/drug effects
Nasopharyngeal Neoplasms/*drug therapy/metabolism/pathology
Neoplasm Invasiveness/*prevention & control
Phosphorylation/drug effects
Pyridines
ortho-Aminobenzoates/*pharmacology/therapeutic use
Anti-Inflammatory Agents, Non-Steroidal
Hepatocyte Growth Factor
Imidazoles
Pyridines
ortho-Aminobenzoates
Figure
Reference
-
1. Oh JK, Weiderpass E. Infection and cancer: global distribution and burden of diseases. Ann Glob Health. 2014; 80:384–392.
Article2. Li K, Lin GZ, Shen JC, Zhou Q. Time trends of nasopharyngeal carcinoma in urban Guangzhou over a 12-year period (2000-2011): declines in both incidence and mortality. Asian Pac J Cancer Prev. 2014; 15:9899–9903.
Article3. Lee AW, Sze WM, Au JS, Leung SF, Leung TW, Chua DT, et al. Treatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experience. Int J Radiat Oncol Biol Phys. 2005; 61:1107–1116.
Article4. Na'ara S, Amit M, Billan S, Cohen JT, Gil Z. Outcome of patients undergoing salvage surgery for recurrent nasopharyngeal carcinoma: a meta-analysis. Ann Surg Oncol. 2014; 21:3056–3062.5. Chan JY, Tsang RK, Wei WI. Morbidities after maxillary swing nasopharyngectomy for recurrent nasopharyngeal carcinoma. Head Neck. 2015; 37:487–492.
Article6. Li JX, Huang SM, Jiang XH, Ouyang B, Han F, Liu S, et al. Local failure patterns for patients with nasopharyngeal carcinoma after intensity-modulated radiotherapy. Radiat Oncol. 2014; 9:87.
Article7. Steward WP, Brown K. Cancer chemoprevention: a rapidly evolving field. Br J Cancer. 2013; 109:1–7.
Article8. Chang JW, Kang SU, Choi JW, Shin YS, Baek SJ, Lee SH, et al. Tolfenamic acid induces apoptosis and growth inhibition in anaplastic thyroid cancer: involvement of nonsteroidal anti-inflammatory drug-activated gene-1 expression and intracellular reactive oxygen species generation. Free Radic Biol Med. 2014; 67:115–130.
Article9. Kang SU, Shin YS, Hwang HS, Baek SJ, Lee SH, Kim CH. Tolfenamic acid induces apoptosis and growth inhibition in head and neck cancer: involvement of NAG-1 expression. PLoS One. 2012; 7:e34988.
Article10. Jeong JB, Choi J, Baek SJ, Lee SH. Reactive oxygen species mediate tolfenamic acid-induced apoptosis in human colorectal cancer cells. Arch Biochem Biophys. 2013; 537:168–175.
Article11. Shin HA, Shin YS, Kang SU, Kim JH, Oh YT, Park KH, et al. Radioprotective effect of epicatechin in cultured human fibroblasts and zebrafish. J Radiat Res. 2014; 55:32–40.
Article12. Lee BS, Kang S, Kim KA, Song YJ, Cheong KH, Cha HY, et al. Met degradation by SAIT301, a Met monoclonal antibody, reduces the invasion and migration of nasopharyngeal cancer cells via inhibition of EGR-1 expression. Cell Death Dis. 2014; 5:e1159.
Article13. Choi MJ, Cho KH, Lee S, Bae YJ, Jeong KJ, Rha SY, et al. hTERT mediates norepinephrine-induced Slug expression and ovarian cancer aggressiveness. Oncogene. 2015; 34:3402–3412.
Article14. Zhao X, Sun B, Sun D, Liu T, Che N, Gu Q, et al. Slug promotes hepatocellular cancer cell progression by increasing sox2 and nanog expression. Oncol Rep. 2015; 33:149–156.
Article15. Merikallio H, T TT, Pääkkö P, Mäkitaro R, Kaarteenaho R, Lehtonen S, et al. Slug is associated with poor survival in squamous cell carcinoma of the lung. Int J Clin Exp Pathol. 2014; 7:5846–5854.16. Wang N, Dong CR, Jiang R, Tang C, Yang L, Jiang QF, et al. Overexpression of HIF-1α, metallothionein and SLUG is associated with high TNM stage and lymph node metastasis in papillary thyroid carcinoma. Int J Clin Exp Pathol. 2013; 7:322–330.17. Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006; 15:1765–1777.
Article18. Tsang J, Lee VH, Kwong DL. Novel therapy for nasopharyngeal carcinoma--where are we. Oral Oncol. 2014; 50:798–801.19. Chiang AK, Mak NK, Ng WT. Translational research in nasopharyngeal carcinoma. Oral Oncol. 2014; 50:345–352.
Article20. You B, Le Tourneau C, Chen EX, Wang L, Jarvi A, Bharadwaj RR, et al. A Phase II trial of erlotinib as maintenance treatment after gemcitabine plus platinum-based chemotherapy in patients with recurrent and/or metastatic nasopharyngeal carcinoma. Am J Clin Oncol. 2012; 35:255–260.
Article21. Chan AT, Hsu MM, Goh BC, Hui EP, Liu TW, Millward MJ, et al. Multicenter, phase II study of cetuximab in combination with carboplatin in patients with recurrent or metastatic nasopharyngeal carcinoma. J Clin Oncol. 2005; 23:3568–3576.
Article22. Kim HS, Lee JH, Park HS, Lee GS, Kim HW, Ha KT, et al. Schizandra chinensis extracts induce apoptosis in human gastric cancer cells via JNK/p38 MAPK activation and the ROS-mediated/mitochondria-dependent pathway. Pharm Biol. 2015; 53:212–219.
Article23. Zhao B, Li X. Altholactone induces reactive oxygen species-mediated apoptosis in bladder cancer T24 cells through mitochondrial dysfunction, MAPK-p38 activation and Akt suppression. Oncol Rep. 2014; 31:2769–2775.
Article24. Kang N, Wang MM, Wang YH, Zhang ZN, Cao HR, Lv YH, et al. Tetrahydrocurcumin induces G2/M cell cycle arrest and apoptosis involving p38 MAPK activation in human breast cancer cells. Food Chem Toxicol. 2014; 67:193–200.
Article25. Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009; 9:537–549.
Article26. Chiacchiera F, Simone C. Signal-dependent regulation of gene expression as a target for cancer treatment: inhibiting p38alpha in colorectal tumors. Cancer Lett. 2008; 265:16–26.
Article27. Sui X, Kong N, Ye L, Han W, Zhou J, Zhang Q, et al. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014; 344:174–179.
Article28. Matrone A, Grossi V, Chiacchiera F, Fina E, Cappellari M, Caringella AM, et al. p38alpha is required for ovarian cancer cell metabolism and survival. Int J Gynecol Cancer. 2010; 20:203–211.29. Grossi V, Simone C. Special Agents Hunting Down Women Silent Killer: The Emerging Role of the p38α Kinase. J Oncol. 2012; 2012:382159.30. Bakin AV, Rinehart C, Tomlinson AK, Arteaga CL. p38 mitogen-activated protein kinase is required for TGFbeta-mediated fibroblastic transdifferentiation and cell migration. J Cell Sci. 2002; 115(Pt 15):3193–3206.
Article31. Hsieh YH, Wu TT, Huang CY, Hsieh YS, Hwang JM, Liu JY. p38 mitogen-activated protein kinase pathway is involved in protein kinase Calpha-regulated invasion in human hepatocellular carcinoma cells. Cancer Res. 2007; 67:4320–4327.
Article32. Lim JH, Woo SM, Min KJ, Park EJ, Jang JH, Seo BR, et al. Rottlerin induces apoptosis of HT29 colon carcinoma cells through NAG-1 upregulation via an ERK and p38 MAPK-dependent and PKC δ-independent mechanism. Chem Biol Interact. 2012; 197:1–7.
Article33. Sharma-Walia N, Patel K, Chandran K, Marginean A, Bottero V, Kerur N, et al. COX-2/PGE2: molecular ambassadors of Kaposi's sarcoma-associated herpes virus oncoprotein-v-FLIP. Oncogenesis. 2012; 1:e5.
Article34. Hemavathy K, Ashraf SI, Ip YT. Snail/slug family of repressors: slowly going into the fast lane of development and cancer. Gene. 2000; 257:1–12.
Article35. Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007; 7:415–428.
Article36. Moody SE, Perez D, Pan TC, Sarkisian CJ, Portocarrero CP, Sterner CJ, et al. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005; 8:197–209.
Article37. Ciruna B, Rossant J. FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev Cell. 2001; 1:37–49.
Article38. Lu Z, Ghosh S, Wang Z, Hunter T. Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of beta-catenin, and enhanced tumor cell invasion. Cancer Cell. 2003; 4:499–515.
Article39. Wang Y, Shi J, Chai K, Ying X, Zhou BP. The Role of Snail in EMT and Tumorigenesis. Curr Cancer Drug Targets. 2013; 13:963–972.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Kinesin superfamily member 15 knockdown inhibits cell proliferation, migration, and invasion in nasopharyngeal carcinoma
- Alisol A Inhibited the Proliferation, Migration, and Invasion of Nasopharyngeal Carcinoma Cells by Inhibiting the Hippo Signaling Pathway
- Met inactivation by S-allylcysteine suppresses the migration and invasion of nasopharyngeal cancer cells induced by hepatocyte growth factor
- Lysophosphatidic Acid-Induced TWIST1 and Slug Expression in Oral Cancer Cell Invasion
- Lobeglitazone, A Peroxisome Proliferator-Activated Receptor-Gamma Agonist, Inhibits Papillary Thyroid Cancer Cell Migration and Invasion by Suppressing p38 MAPK Signaling Pathway