Endocrinol Metab.
2021 Oct;36(5):1095-1110. 10.3803/EnM.2021.1155.
Lobeglitazone, A Peroxisome Proliferator-Activated Receptor-Gamma Agonist, Inhibits Papillary Thyroid Cancer Cell Migration and Invasion by Suppressing p38 MAPK Signaling Pathway
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2521957
- DOI: http://doi.org/10.3803/EnM.2021.1155
Abstract
- Background
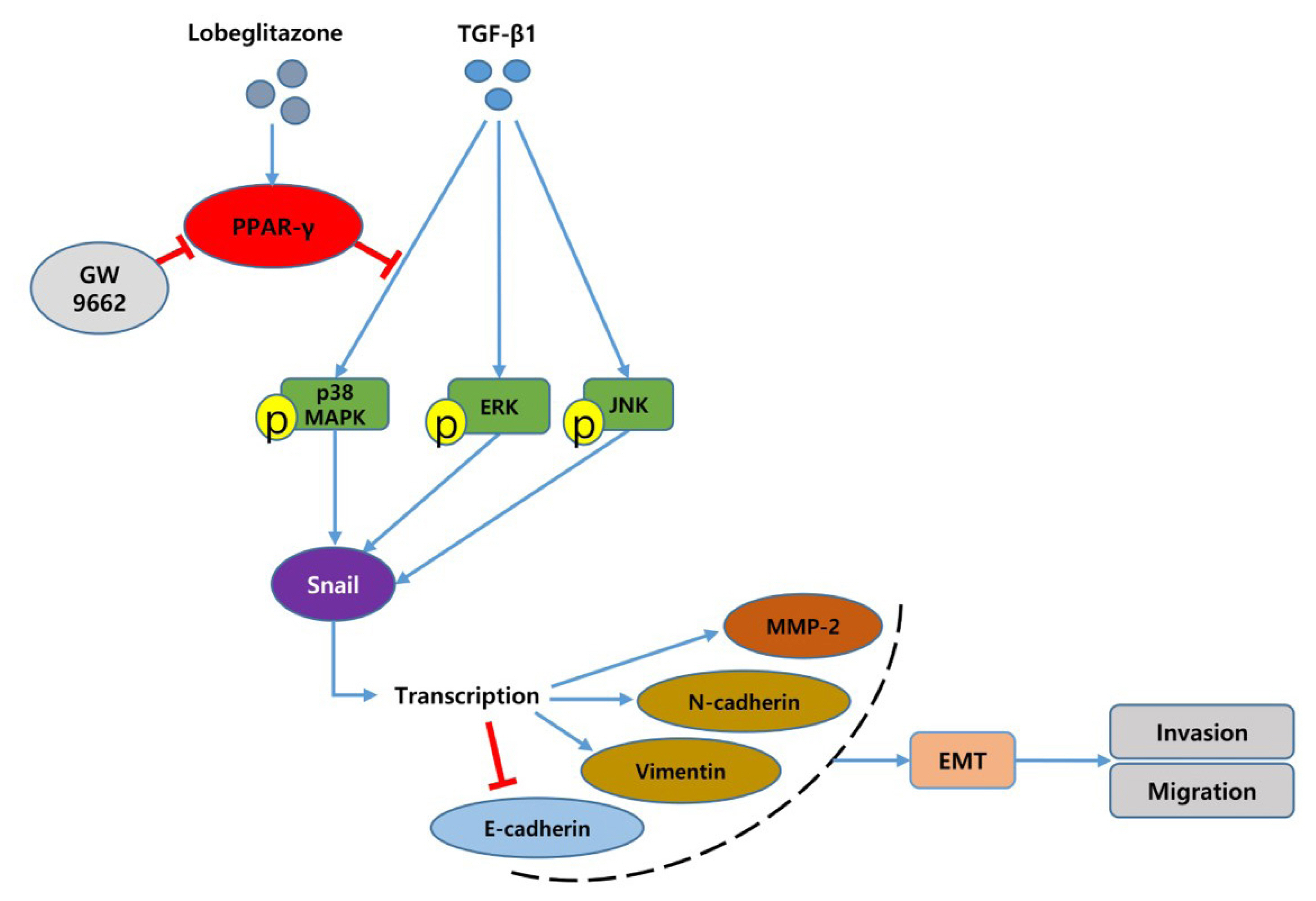
Peroxisome proliferator-activated receptor-gamma (PPAR-γ) ligands have been widely shown to correlate with epithelial-mesenchymal transition (EMT) and cancer progression. Lobeglitazone (LGZ) is a novel ligand of PPAR-γ; and its role in EMT and metastasis in papillary thyroid carcinoma (PTC) is poorly understood. We aimed to investigate the role of LGZ in metastatic behavior of PTC cells.
Methods
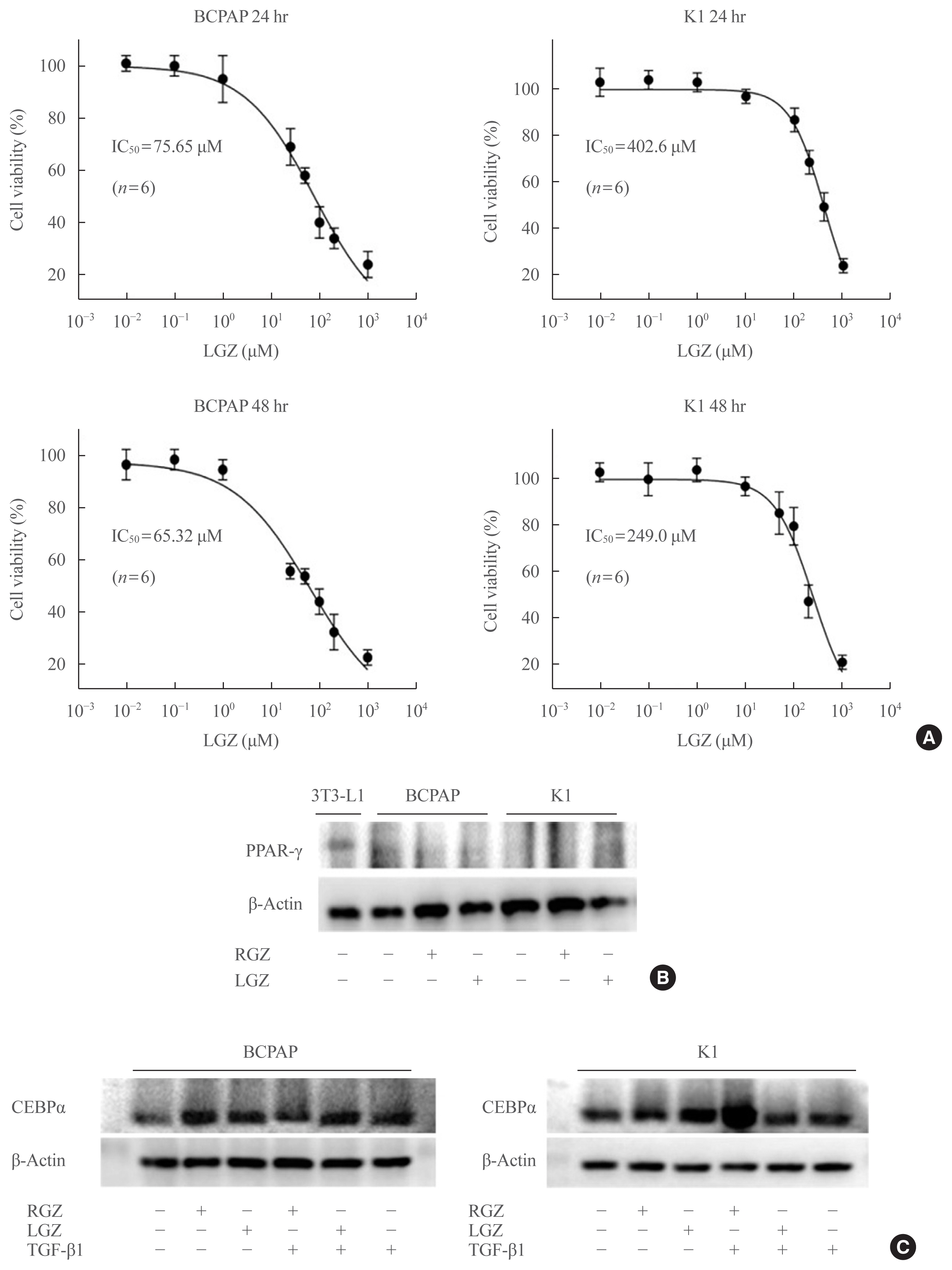
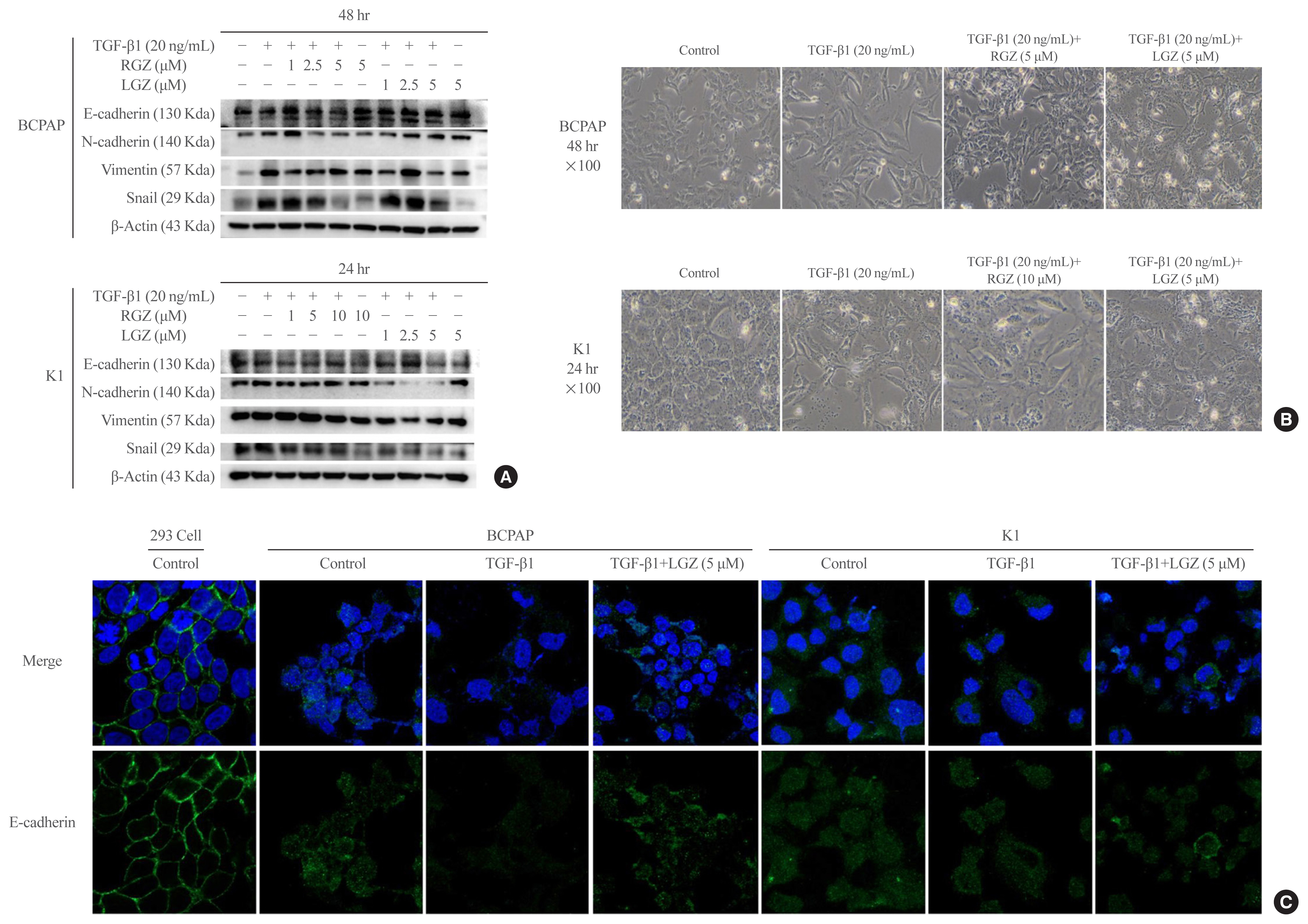
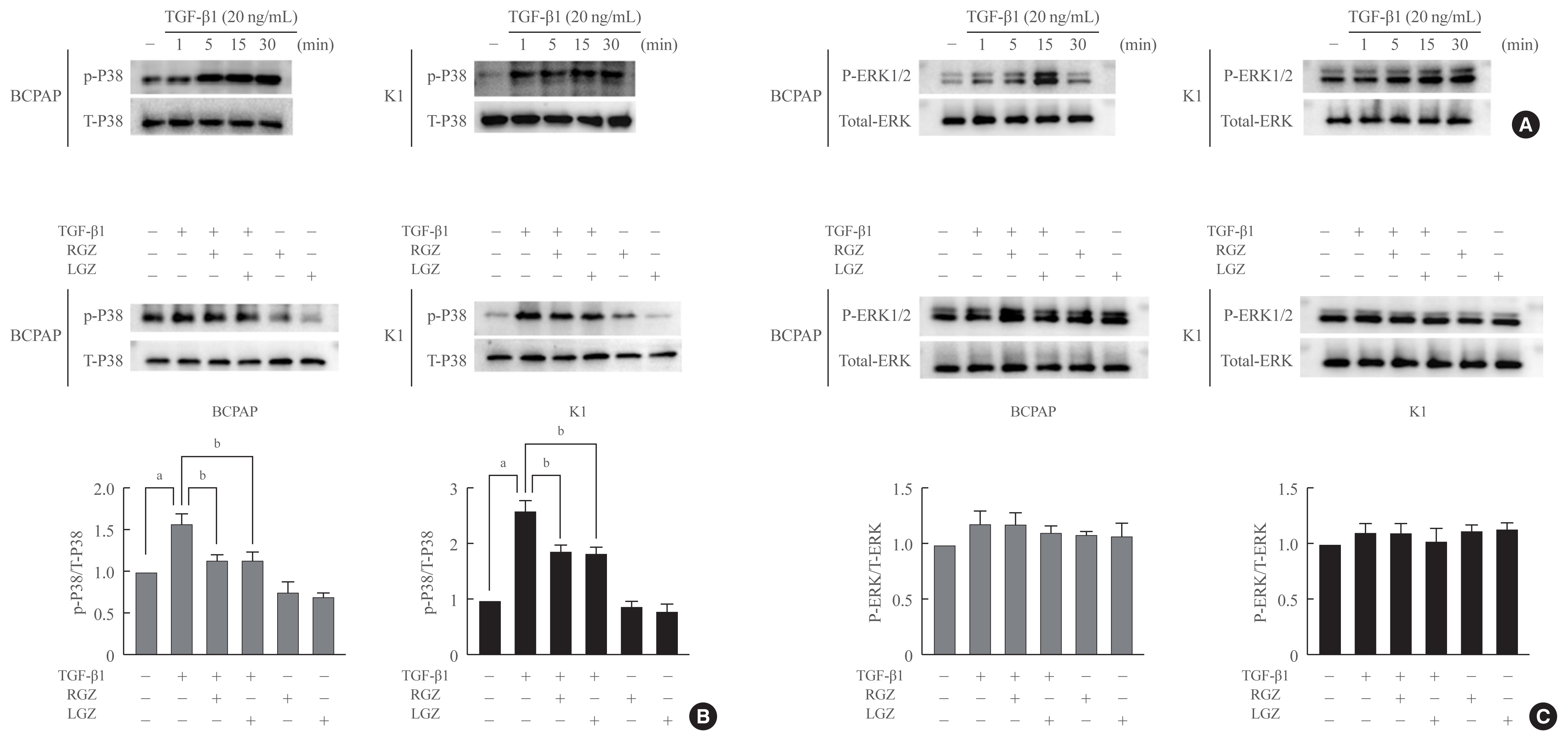
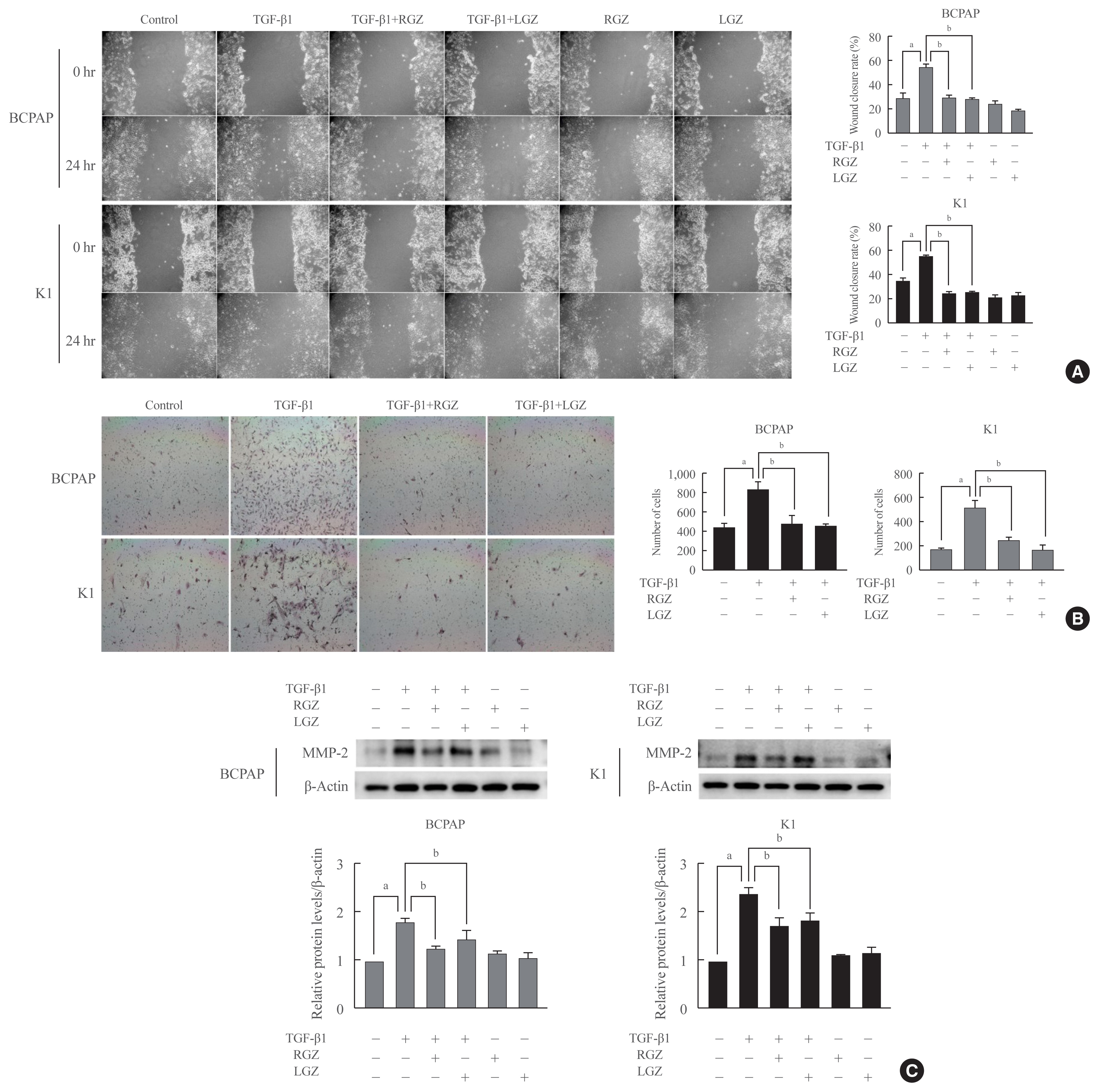
Half maximal inhibitory concentration (IC50) values of LGZ in BRAF-mutated PTC cell lines (BCPAP and K1) were determined using MTT assay. Rosiglitazone (RGZ), the PPAR-γ ligand was used as a positive control. The protein expression of PPAR-γ, cell-surface proteins (E-cadherin, N-cadherin), cytoskeletal protein (Vimentin), transcription factor (Snail), p38 mitogenactivated protein kinase (MAPK), extracellular signal-regulated kinase (ERK) 1/2 pathway, and matrix metalloproteinase (MMP)-2 expression were measured using Western blotting. Changes in E-cadherin expression were also determined using immunocytochemistry. Cell migration and invasion were analyzed using wound healing and Matrigel invasion assays.
Results
Treatment with LGZ or RGZ significantly inhibited transforming growth factor-beta1 (TGF-β1)-induced EMT-associated processes such as fibroblast-like morphological changes, EMT-related protein expression, and increased cell migration and invasion in BCPAP and K1 cells. LGZ restored TGF-β1-induced loss of E-cadherin, as observed using immunocytochemistry. Furthermore, LGZ and RGZ suppressed TGF-β1-induced MMP-2 expression and phosphorylation of p38 MAPK, but not ERK1/2. Although there was no change in PPAR-γ expression after treatment with LGZ or RGZ, the effect of downstream processes mediated by LGZ was hampered by GW9662, a PPAR-γ antagonist.
Conclusion
LGZ inhibits TGF-β1-induced EMT, migration, and invasion through the p38 MAPK signaling pathway in a PPAR-γ-dependent manner in PTC cells.
Keyword
Figure
Reference
-
1. Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol. 2013; 2013:965212.
Article2. Lubitz CC, Sosa JA. The changing landscape of papillary thyroid cancer: epidemiology, management, and the implications for patients. Cancer. 2016; 122:3754–9.
Article3. Morris LG, Tuttle RM, Davies L. Changing trends in the incidence of thyroid cancer in the United States. JAMA Otolaryngol Head Neck Surg. 2016; 142:709–11.
Article4. LiVolsi VA. Papillary thyroid carcinoma: an update. Mod Pathol. 2011; 24(Suppl 2):S1–9.
Article5. Lebastchi AH, Callender GG. Thyroid cancer. Curr Probl Cancer. 2014; 38:48–74.
Article6. Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009; 19:156–72.7. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009; 139:871–90.
Article8. Wei SC, Fattet L, Yang J. The forces behind EMT and tumor metastasis. Cell Cycle. 2015; 14:2387–8.
Article9. Vu T, Datta PK. Regulation of EMT in colorectal cancer: a culprit in metastasis. Cancers (Basel). 2017; 9:171.
Article10. Javelaud D, Mauviel A. Mammalian transforming growth factor-betas: Smad signaling and physio-pathological roles. Int J Biochem Cell Biol. 2004; 36:1161–5.11. Vasko V, Espinosa AV, Scouten W, He H, Auer H, Liyanarachchi S, et al. Gene expression and functional evidence of epithelial-to-mesenchymal transition in papillary thyroid carcinoma invasion. Proc Natl Acad Sci U S A. 2007; 104:2803–8.
Article12. Riesco-Eizaguirre G, Rodriguez I, De la Vieja A, Costamagna E, Carrasco N, Nistal M, et al. The BRAFV600E oncogene induces transforming growth factor beta secretion leading to sodium iodide symporter repression and increased malignancy in thyroid cancer. Cancer Res. 2009; 69:8317–25.13. Knauf JA, Sartor MA, Medvedovic M, Lundsmith E, Ryder M, Salzano M, et al. Progression of BRAF-induced thyroid cancer is associated with epithelial-mesenchymal transition requiring concomitant MAP kinase and TGFβ signaling. Oncogene. 2011; 30:3153–62.
Article14. Atfi A, Djelloul S, Chastre E, Davis R, Gespach C. Evidence for a role of Rho-like GTPases and stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) in transforming growth factor beta-mediated signaling. J Biol Chem. 1997; 272:1429–32.15. Fu H, He Y, Qi L, Chen L, Luo Y, Chen L, et al. cPLA2α activates PI3K/AKT and inhibits Smad2/3 during epithelial-mesenchymal transition of hepatocellular carcinoma cells. Cancer Lett. 2017; 403:260–70.
Article16. Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997; 91:439–42.
Article17. Aschebrook-Kilfoy B, Sabra MM, Brenner A, Moore SC, Ron E, Schatzkin A, et al. Diabetes and thyroid cancer risk in the National Institutes of Health-AARP Diet and Health Study. Thyroid. 2011; 21:957–63.
Article18. Hsu IR, Kim SP, Kabir M, Bergman RN. Metabolic syndrome, hyperinsulinemia, and cancer. Am J Clin Nutr. 2007; 86:s867–71.
Article19. Tseng CH. Rosiglitazone may reduce thyroid cancer risk in patients with type 2 diabetes. Ann Med. 2013; 45:539–44.
Article20. Grommes C, Landreth GE, Heneka MT. Antineoplastic effects of peroxisome proliferator-activated receptor gamma agonists. Lancet Oncol. 2004; 5:419–29.21. Reka AK, Kurapati H, Narala VR, Bommer G, Chen J, Standiford TJ, et al. Peroxisome proliferator-activated receptor-gamma activation inhibits tumor metastasis by antagonizing Smad3-mediated epithelial-mesenchymal transition. Mol Cancer Ther. 2010; 9:3221–32.22. Elstner E, Muller C, Koshizuka K, Williamson EA, Park D, Asou H, et al. Ligands for peroxisome proliferator-activated receptorgamma and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Proc Natl Acad Sci U S A. 1998; 95:8806–11.23. Giordano TJ, Haugen BR, Sherman SI, Shah MH, Caoili EM, Koenig RJ. Pioglitazone therapy of PAX8-PPARγ fusion protein thyroid carcinoma. J Clin Endocrinol Metab. 2018; 103:1277–81.
Article24. Kato Y, Ying H, Zhao L, Furuya F, Araki O, Willingham MC, et al. PPARgamma insufficiency promotes follicular thyroid carcinogenesis via activation of the nuclear factor-kappaB signaling pathway. Oncogene. 2006; 25:2736–47.
Article25. Aiello A, Pandini G, Frasca F, Conte E, Murabito A, Sacco A, et al. Peroxisomal proliferator-activated receptor-gamma agonists induce partial reversion of epithelial-mesenchymal transition in anaplastic thyroid cancer cells. Endocrinology. 2006; 147:4463–75.26. Lee JH, Noh CK, Yim CS, Jeong YS, Ahn SH, Lee W, et al. Kinetics of the absorption, distribution, metabolism, and excretion of lobeglitazone, a novel activator of peroxisome proliferator-activated receptor gamma in rats. J Pharm Sci. 2015; 104:3049–59.
Article27. Moon KS, Lee JE, Lee HS, Hwang IC, Kim DH, Park HK, et al. CKD-501, a novel selective PPARγ agonist, shows no carcinogenic potential in ICR mice following oral administration for 104 weeks. J Appl Toxicol. 2014; 34:1271–84.
Article28. Lee HS, Chang M, Lee JE, Kim W, Hwang IC, Kim DH, et al. Carcinogenicity study of CKD-501, a novel dual peroxisome proliferator-activated receptors α and γ agonist, following oral administration to Sprague Dawley rats for 94–101 weeks. Regul Toxicol Pharmacol. 2014; 69:207–16.29. Nemenoff RA. Peroxisome proliferator-activated receptor-gamma in lung cancer: defining specific versus “off-target” effectors. J Thorac Oncol. 2007; 2:989–92.30. Han S, Roman J. Rosiglitazone suppresses human lung carcinoma cell growth through PPARgamma-dependent and PPARgamma-independent signal pathways. Mol Cancer Ther. 2006; 5:430–7.31. Bae KH, Seo JB, Jung YA, Seo HY, Kang SH, Jeon HJ, et al. Lobeglitazone, a novel peroxisome proliferator-activated receptor γ agonist, attenuates renal fibrosis caused by unilateral ureteral obstruction in mice. Endocrinol Metab (Seoul). 2017; 32:115–23.
Article32. Vella V, Nicolosi ML, Giuliano S, Bellomo M, Belfiore A, Malaguarnera R. PPAR-γ agonists as antineoplastic agents in cancers with dysregulated IGF axis. Front Endocrinol (Lausanne). 2017; 8:31.
Article33. Feinstein DL, Spagnolo A, Akar C, Weinberg G, Murphy P, Gavrilyuk V, et al. Receptor-independent actions of PPAR thiazolidinedione agonists: is mitochondrial function the key? Biochem Pharmacol. 2005; 70:177–88.
Article34. Wood WM, Sharma V, Bauerle KT, Pike LA, Zhou Q, Fretwell DL, et al. PPARγ promotes growth and invasion of thyroid cancer cells. PPAR Res. 2011; 2011:171765.35. Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994; 79:1147–56.
Article36. Sugii S, Olson P, Sears DD, Saberi M, Atkins AR, Barish GD, et al. PPARgamma activation in adipocytes is sufficient for systemic insulin sensitization. Proc Natl Acad Sci U S A. 2009; 106:22504–9.37. Lefterova MI, Zhang Y, Steger DJ, Schupp M, Schug J, Cristancho A, et al. PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008; 22:2941–52.38. Miller M, Shuman JD, Sebastian T, Dauter Z, Johnson PF. Structural basis for DNA recognition by the basic region leucine zipper transcription factor CCAAT/enhancer-binding protein alpha. J Biol Chem. 2003; 278:15178–84.39. Dobson ME, Diallo-Krou E, Grachtchouk V, Yu J, Colby LA, Wilkinson JE, et al. Pioglitazone induces a proadipogenic antitumor response in mice with PAX8-PPARgamma fusion protein thyroid carcinoma. Endocrinology. 2011; 152:4455–65.
Article40. Sato A, Yamada N, Ogawa Y, Ikegami M. CCAAT/enhancer-binding protein-α suppresses lung tumor development in mice through the p38α MAP kinase pathway. PLoS One. 2013; 8:e57013.
Article41. Lu J, Du C, Yao J, Wu B, Duan Y, Zhou L, et al. C/EBPα suppresses lung adenocarcinoma cell invasion and migration by inhibiting β-catenin. Cell Physiol Biochem. 2017; 42:1779–88.
Article42. Saiselet M, Floor S, Tarabichi M, Dom G, Hebrant A, van Staveren WC, et al. Thyroid cancer cell lines: an overview. Front Endocrinol (Lausanne). 2012; 3:133.
Article43. Lourenco AR, Roukens MG, Seinstra D, Frederiks CL, Pals CE, Vervoort SJ, et al. C/EBPα is crucial determinant of epithelial maintenance by preventing epithelial-to-mesenchymal transition. Nat Commun. 2020; 11:785.
Article44. Qi C, Zhu Y, Reddy JK. Peroxisome proliferator-activated receptors, coactivators, and downstream targets. Cell Biochem Biophys. 2000; 32:187–204.
Article45. Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008; 68:3645–54.
Article46. Lee HJ, Su Y, Yin PH, Lee HC, Chi CW. PPAR(gamma)/PGC-1(alpha) pathway in E-cadherin expression and motility of HepG2 cells. Anticancer Res. 2009; 29:5057–63.47. Chang SN, Lee JM, Oh H, Kim U, Ryu B, Park JH. Troglitazone inhibits the migration and invasion of PC-3 human prostate cancer cells by upregulating E-cadherin and glutathione peroxidase 3. Oncol Lett. 2018; 16:5482–8.
Article48. Zhou Q, Chen J, Feng J, Xu Y, Zheng W, Wang J. SOSTDC1 inhibits follicular thyroid cancer cell proliferation, migration, and EMT via suppressing PI3K/Akt and MAPK/Erk signaling pathways. Mol Cell Biochem. 2017; 435:87–95.
Article49. Tse JC, Kalluri R. Mechanisms of metastasis: epithelial-to-mesenchymal transition and contribution of tumor microenvironment. J Cell Biochem. 2007; 101:816–29.
Article50. Palona I, Namba H, Mitsutake N, Starenki D, Podtcheko A, Sedliarou I, et al. BRAFV600E promotes invasiveness of thyroid cancer cells through nuclear factor kappaB activation. Endocrinology. 2006; 147:5699–707.
Article51. Zou M, Baitei EY, BinEssa HA, Al-Mohanna FA, Parhar RS, St-Arnaud R, et al. Cyp24a1 attenuation limits progression of BrafV600E-induced papillary thyroid cancer cells and sensitizes them to BRAFV600E inhibitor PLX4720. Cancer Res. 2017; 77:2161–72.52. Jorda M, Olmeda D, Vinyals A, Valero E, Cubillo E, Llorens A, et al. Upregulation of MMP-9 in MDCK epithelial cell line in response to expression of the Snail transcription factor. J Cell Sci. 2005; 118(Pt 15):3371–85.
Article53. Xie L, Law BK, Chytil AM, Brown KA, Aakre ME, Moses HL. Activation of the Erk pathway is required for TGF-beta1-induced EMT in vitro. Neoplasia. 2004; 6:603–10.
Article54. Yu L, Hebert MC, Zhang YE. TGF-beta receptor-activated p38 MAP kinase mediates Smad-independent TGF-beta responses. EMBO J. 2002; 21:3749–59.
Article55. Kumar B, Koul S, Petersen J, Khandrika L, Hwa JS, Meacham RB, et al. p38 mitogen-activated protein kinase-driven MAPKAPK2 regulates invasion of bladder cancer by modulation of MMP-2 and MMP-9 activity. Cancer Res. 2010; 70:832–41.
Article56. Chapnick DA, Warner L, Bernet J, Rao T, Liu X. Partners in crime: the TGFβ and MAPK pathways in cancer progression. Cell Biosci. 2011; 1:42.
Article57. Ciaramella V, Sasso FC, Di Liello R, Corte CM, Barra G, Viscardi G, et al. Activity and molecular targets of pioglitazone via blockade of proliferation, invasiveness and bioenergetics in human NSCLC. J Exp Clin Cancer Res. 2019; 38:178.
Article58. Nuwormegbe SA, Sohn JH, Kim SW. A PPAR-gamma agonist rosiglitazone suppresses fibrotic response in human pterygium fibroblasts by modulating the p38 MAPK pathway. Invest Ophthalmol Vis Sci. 2017; 58:5217–26.
Article59. Poleni PE, Bianchi A, Etienne S, Koufany M, Sebillaud S, Netter P, et al. Agonists of peroxisome proliferators-activated receptors (PPAR) alpha, beta/delta or gamma reduce transforming growth factor (TGF)-beta-induced proteoglycans’ production in chondrocytes. Osteoarthritis Cartilage. 2007; 15:493–505.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Caspase Recruitment Domain Containing Protein 9 Suppresses Non-Small Cell Lung Cancer Proliferation and Invasion via Inhibiting MAPK/p38 Pathway
- Expression of Down Stream Molecules of RET (p-ERK, p-p38 MAPK, p-JNK and p-AKT) in Papillary Thyroid Carcinomas
- Role of Pexoxisome Proliferator Activated Receptor Gamma in Growth Regulation of Thyroid Cancer Cells
- Ras Mitogen-activated Protein Kinase Signaling and Kinase Suppressor of Ras as Therapeutic Targets for Hepatocellular Carcinoma
- Effect of Transforming Growth Factor (TGF)-beta and Peroxisome Proliferator-Activated Receptor (PPAR)-gamma in Endometrial Decidualization