Ann Lab Med.
2016 May;36(3):230-234. 10.3343/alm.2016.36.3.230.
External Quality Assessment of MERS-CoV Molecular Diagnostics During the 2015 Korean Outbreak
- Affiliations
-
- 1Department of Laboratory Medicine, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, Korea. sparkle@snu.ac.kr
- 2Department of Laboratory Medicine, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- 3Department of Laboratory Medicine, Kangdong Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea.
- KMID: 2373532
- DOI: http://doi.org/10.3343/alm.2016.36.3.230
Abstract
- BACKGROUND
The largest outbreak of Middle East respiratory syndrome coronavirus (MERS-CoV) infection outside Middle East Asia in 2015 has necessitated the rapid expansion of laboratories that conduct MERS-CoV molecular testing in Korea, together with external quality assessment (EQA) to evaluate the assays used.
METHODS
The EQA program consisted of two phases; self-validation and blind assessment. For the first EQA phase, in vitro transcribed upstream region of the envelope gene (upE) and the open reading frame (ORF)1a RNAs were used at a concentration of 1,000 copies/microL. The test panel for the second EQA phase consisted of RNA extracts from three samples, which were obtained from two MERS-CoV positive patients and one MERS-CoV negative patient.
RESULTS
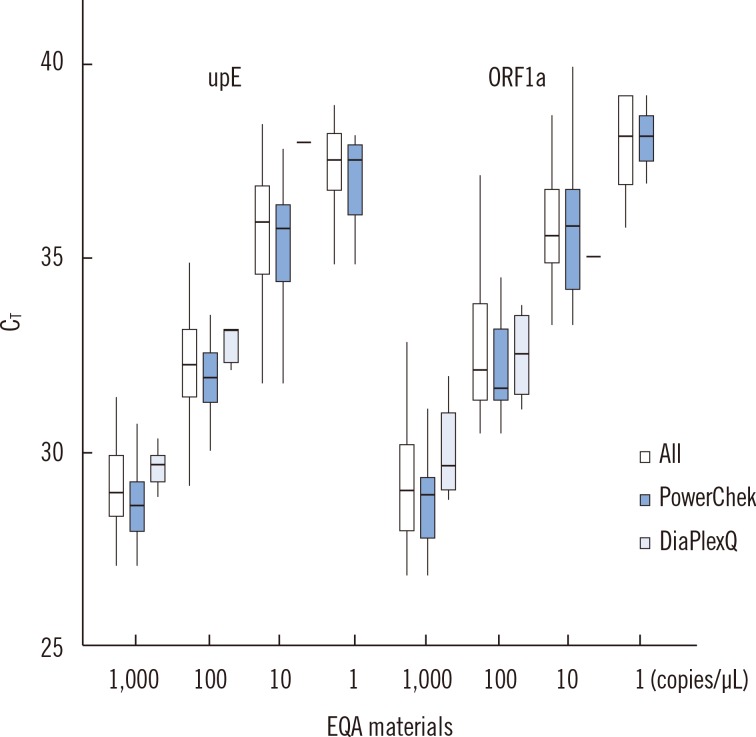
The first EQA phase results for 46 participants showed a linear relationship between the threshold cycle (CT) values of RNA materials and the logarithmic concentrations for both upE and ORF1a gene targets (R2=0.73 and 0.75, respectively). The mean CT value for each concentration was different depending on which commercial kit was used for the assay. Among the three commonly used kits, PowerChek MERS Real-Time PCR kit (KogeneBiotech, Korea) showed the lowest CT values at all concentrations of upE and most concentrations of ORF1a. The second EQA phase results for 47 participants were 100% correct for all tested samples.
CONCLUSIONS
This EQA survey demonstrates that the MERS-CoV molecular testing performed in Korea during the 2015 outbreak is of robust capability. However, careful establishment and validation of a cut-off value are recommended to ensure good analytical sensitivity.
MeSH Terms
-
Coronavirus Infections/*diagnosis/epidemiology/virology
Disease Outbreaks
Humans
Middle East Respiratory Syndrome Coronavirus/*genetics/isolation & purification
Molecular Diagnostic Techniques/*standards
Quality Assurance, Health Care
RNA, Viral/analysis
Real-Time Polymerase Chain Reaction
Republic of Korea/epidemiology
Surveys and Questionnaires
RNA, Viral
Figure
Reference
-
1. van Boheemen S, de Graaf M, Lauber C, Bestebroer TM, Raj VS, Zaki AM, et al. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. MBio. 2012; 3:pii.e00473-12.
Article2. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012; 367:1814–1820. PMID: 23075143.
Article3. Corman VM, Eckerle I, Bleicker T, Zaki A, Landt O, Eschbach-Bludau M, et al. Detection of a novel human coronavirus by real-time reverse-transcription polymerase chain reaction. Euro Surveill. 2012; 17:pii:20285.
Article4. Corman VM, Muller MA, Costabel U, Timm J, Binger T, Meyer B, et al. Assays for laboratory confirmation of novel human coronavirus (hCoV-EMC) infections. Euro Surveill. 2012; 17:pii:20334.
Article5. WHO. Laboratory Testing for Middle East respiratory syndrome coronavirus. Interim recommendations (revised). Updated on Sep 2014. http://www.who.int/csr/disease/coronavirus_infections/WHO_interim_recommendations_lab_detection_MERSCoV_092014.pdf.6. Korean Society of Infectious Diseases. Korean Society for Healthcare-associated Infection Control and Prevention. An unexpected outbreak of Middle East respiratory syndrome coronavirus infection in the Republic of Korea, 2015. Infect Chemother. 2015; 47:120–122. PMID: 26157591.7. WHO. Middle East respiratory syndrome coronavirus (MERS-CoV)-Republic of Korea. Accessed on Nov 2015. http://www.who.int/csr/don/25-october-2015-mers-korea/en/.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Same Middle East Respiratory Syndrome-Coronavirus (MERS-CoV) yet Different Outbreak Patterns and Public Health Impacts on the Far East Expert Opinion from the Rapid Response Team of the Republic of Korea
- Current Status of Proficiency Testing Programs and Development of Reference Materials for Severe Acute Respiratory Syndrome Coronavirus 2 Molecular Diagnostic Tests
- Middle East respiratory syndrome: what we learned from the 2015 outbreak in the Republic of Korea
- Current Advances in the Development of Vaccines and Therapeutic Agents Against MERS-coV
- Lessons Learned from SARS-CoV and MERS-CoV: Preparation for SARS-CoV-2 induced COVID-19