Ann Lab Med.
2016 Mar;36(2):197-201. 10.3343/alm.2016.36.2.197.
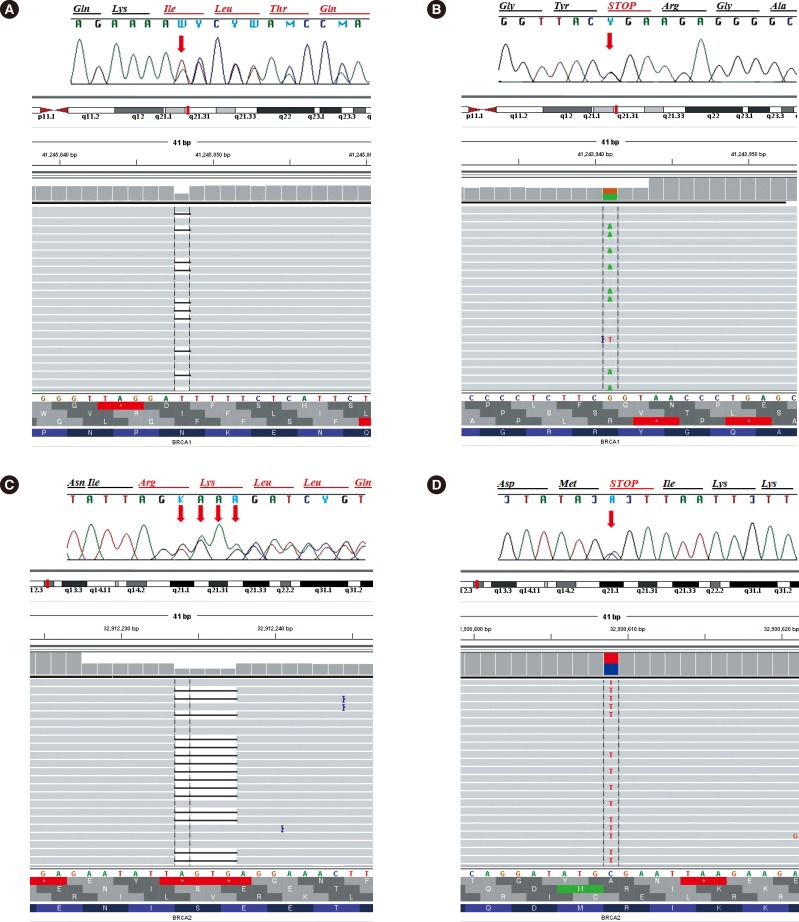
Comparison of Targeted Next-Generation and Sanger Sequencing for the BRCA1 and BRCA2 Mutation Screening
- Affiliations
-
- 1Department of Laboratory Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea. microkim@catholic.ac.kr
- 2Catholic Genetic Laboratory Center, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Samkwang Medical Laboratories, Seoul, Korea. chumgang@smlab.co.kr
- KMID: 2373527
- DOI: http://doi.org/10.3343/alm.2016.36.2.197
Abstract
- No abstract available.
MeSH Terms
Figure
Cited by 1 articles
-
Comparison of Ion Personal Genome Machine Platforms for the Detection of Variants in
BRCA1 andBRCA2
Sang Mee Hwang, Ki Chan Lee, Min Seob Lee, Kyoung Un Park
Cancer Res Treat. 2018;50(1):255-264. doi: 10.4143/crt.2017.062.
Reference
-
1. Banerjee S, Kaye SB, Ashworth A. Making the best of PARP inhibitors in ovarian cancer. Nat Rev Clin Oncol. 2010; 7:508–519. PMID: 20700108.
Article2. Secord AA, Barnett JC, Ledermann JA, Peterson BL, Myers ER, Havrilesky LJ. Cost-effectiveness of BRCA1 and BRCA2 mutation testing to target PARP inhibitor use in platinum-sensitive recurrent ovarian cancer. Int J Gynecol Cancer. 2013; 23:846–852. PMID: 23666017.3. Yazici H, Glendon G, Yazici H, Burnie SJ, Saip P, Buyru F, et al. BRCA1 and BRCA2 mutations in Turkish familial and non-familial ovarian cancer patients: a high incidence of mutations in non-familial cases. Hum Mutat. 2002; 20:28–34. PMID: 12112655.
Article4. Friedman LS, Ostermeyer EA, Szabo CI, Dowd P, Lynch ED, Rowell SE, et al. Confirmation of BRCA1 by analysis of germline mutations linked to breast and ovarian cancer in ten families. Nat Genet. 1994; 8:399–404. PMID: 7894493.
Article5. Meindl A. Comprehensive analysis of 989 patients with breast or ovarian cancer provides BRCA1 and BRCA2 mutation profiles and frequencies for the German population. Int J Cancer. 2002; 97:472–480. PMID: 11802209.6. Vehmanen P, Friedman LS, Eerola H, McClure M, Ward B, Sarantaus L, et al. Low proportion of BRCA1 and BRCA2 mutations in Finnish breast cancer families: evidence for additional susceptibility genes. Hum Mol Genet. 1997; 6:2309–2315. PMID: 9361038.
Article7. Rothberg JM, Hinz W, Rearick TM, Schultz J, Mileski W, Davey M, et al. An integrated semiconductor device enabling non-optical genome sequencing. Nature. 2011; 475:348–352. PMID: 21776081.
Article8. Bragg LM, Stone G, Butler MK, Hugenholtz P, Tyson GW. Shining a light on dark sequencing: characterising errors in Ion Torrent PGM data. PLoS Comput Biol. 2013; 9:e1003031. PMID: 23592973.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Performance Evaluation of BRCA1/2 Genetic Test Using Next-Generation Sequencing Based on Target Capture Method
- Next-generation sequencing of BRCA1/2 in breast cancer patients: potential effects on clinical decision-making using rapid, high-accuracy genetic results
- Evaluation of a Targeted Next-generation Sequencing Assay for BRCA Mutation Screening in Clinical Samples
- Novel Germline Mutations of BRCA1 and BRCA2 in Korean Familial Breast Cancer Patients
- Frequency of BRCA1 and BRCA2 Germline Mutations Detected by Protein Truncation Test and Cumulative Risks of Breast and Ovarian Cancer among Mutation Carriers in Japanese Breast Cancer Families