Ann Lab Med.
2016 Jan;36(1):28-35. 10.3343/alm.2016.36.1.28.
Basophil Markers for Identification and Activation in the Indirect Basophil Activation Test by Flow Cytometry for Diagnosis of Autoimmune Urticaria
- Affiliations
-
- 1Department of Clinical Pathology, Kyungpook National University School of Medicine, Daegu, Korea. wondi@knu.ac.kr
- 2Department of Pediatrics, Kyungpook National University School of Medicine, Daegu, Korea.
- 3Department of Emergency Medicine, Kyungpook National University School of Medicine, Daegu, Korea.
- KMID: 2373494
- DOI: http://doi.org/10.3343/alm.2016.36.1.28
Abstract
- BACKGROUND
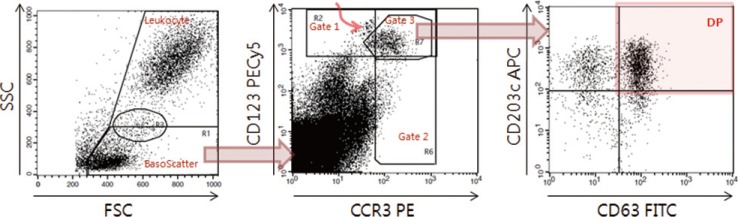
The indirect basophil activation test using flow cytometry is a promising tool for autoimmune urticaria diagnosis. We aimed to identify better donor basophils (from atopic vs. non-atopic donors and interleukin-3 primed vs. unprimed basophils) and improve basophil identification and activation markers (eotaxin CC chemokine receptor-3 [CCR3] vs. CD123 and CD63 vs. CD203c).
METHODS
Donor basophils were obtained from non-atopic and atopic group O donors. Positive control sera were artificially prepared to simulate autoimmune urticaria patients' sera. Patient sera were obtained from nine children with chronic urticaria. Assay sensitivity was compared among each variation by using positive control sera (n=21), applying cutoff values defined from negative control sera (n=20).
RESULTS
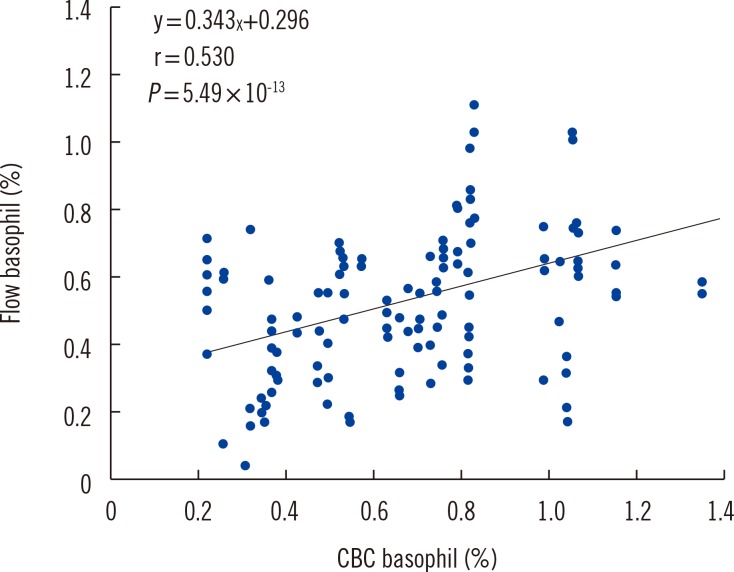
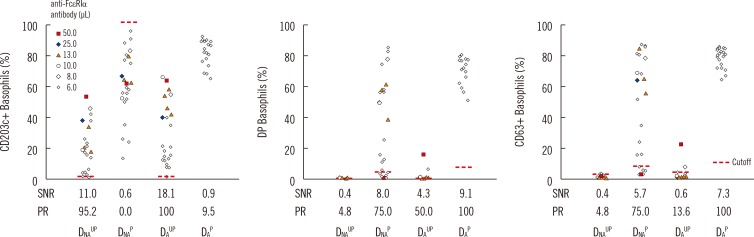
For basophil identification, a combination of CCR3 and CD123 markers revealed a higher correlation with automated complete blood count (r=0.530) compared with that observed using CD123 (r=0.498) or CCR3 alone (r=0.195). Three activation markers on the atopic donor basophils attained 100% assay sensitivity: CD203c on unprimed basophils, CD63+CD203+ or CD63 alone on primed basophils; however, these markers on the non-atopic donor basophils attained lower assay sensitivity.
CONCLUSIONS
For basophil identification markers, a combination of CD123 and CCR3 is recommended, while CD123 alone may be used as an alternative. Donor basophils should be obtained from an atopic donor. For basophil activation markers, either CD203c alone on unprimed basophils or CD203c and CD63 on primed basophils are recommended, while CD63 alone on primed basophils may be used as an alternative.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Reply to the Letter to the Editor by Dr. Chirumbolo
Dong Il Won
Ann Lab Med. 2016;36(5):498-498. doi: 10.3343/alm.2016.36.5.498.Neutrophil oxidative burst as a diagnostic indicator of IgG-mediated anaphylaxis
Dong Il Won, Sujeong Kim, Eun Hee Lee
Blood Res. 2018;53(4):299-306. doi: 10.5045/br.2018.53.4.299.
Reference
-
1. Marrouche N, Grattan C. Childhood urticaria. Curr Opin Allergy Clin Immunol. 2012; 12:485–490. PMID: 22825024.
Article2. Fiebiger E, Hammerschmid F, Stingl G, Maurer D. Anti-FcepsilonRIalpha autoantibodies in autoimmune-mediated disorders. Identification of a structure-function relationship. J Clin Invest. 1998; 101:243–251. PMID: 9421487.
Article3. Irinyi B, Széles G, Gyimesi E, Tumpek J, Herédi E, Dimitrios G, et al. Clinical and laboratory examinations in the subgroups of chronic urticaria. Int Arch Allergy Immunol. 2007; 144:217–225. PMID: 17579280.
Article4. Ebo DG, Bridts CH, Hagendorens MM, Aerts NE, De Clerck LS, Stevens WJ. Basophil activation test by flow cytometry: present and future applications in allergology. Cytometry B Clin Cytom. 2008; 74:201–210. PMID: 18412216.
Article5. Marone G, Spadaro G, Patella V, Genovese A. The clinical relevance of basophil releasability. J Allergy Clin Immunol. 1994; 94:1293–1303. PMID: 7528235.
Article6. Zuberbier T, Schwarz S, Hartmann K, Pfrommer C, Czarnetzki BM. Histamine releasability of basophils and skin mast cells in chronic urticaria. Allergy. 1996; 51:24–28. PMID: 8721524.
Article7. Szegedi A, Irinyi B, Gál M, Hunyadi J, Dankó K, Kiss E, et al. Significant correlation between the CD63 assay and the histamine release assay in chronic urticaria. Br J Dermatol. 2006; 155:67–75. PMID: 16792754.
Article8. Sabroe RA, Grattan CE, Francis DM, Barr RM, Kobza Black A, Greaves MW. The autologous serum skin test: a screening test for autoantibodies in chronic idiopathic urticaria. Br J Dermatol. 1999; 140:446–452. PMID: 10233264.
Article9. Frezzolini A, Provini A, Teofoli P, Pomponi D, De Pità O. Serum-induced basophil CD63 expression by means of a tricolour flow cytometric method for the in vitro diagnosis of chronic urticaria. Allergy. 2006; 61:1071–1077. PMID: 16918509.
Article10. Gyimesi E, Sipka S, Dankó K, Kiss E, Hidvégi B, Gál M, et al. Basophil CD63 expression assay on highly sensitized atopic donor leucocytes-a useful method in chronic autoimmune urticaria. Br J Dermatol. 2004; 151:388–396. PMID: 15327546.
Article11. Ocmant A, Peignois Y, Mulier S, Hanssens L, Michils A, Schandené L. Flow cytometry for basophil activation markers: the measurement of CD203c up-regulation is as reliable as CD63 expression in the diagnosis of cat allergy. J Immunol Methods. 2007; 320:40–48. PMID: 17275019.
Article12. Cozon G, Ferrándiz J, Peyramond D, Brunet J. Detection of activated basophils using flow cytometry for diagnosis in atopic patients. Allergol Immunopathol (Madr). 1999; 27:182–187. PMID: 10486441.13. Ebo DG, Lechkar B, Schuerwegh AJ, Bridts CH, De Clerck LS, Stevens WJ. Comments regarding 'Marked improvement of the basophil activation test by detecting CD203c instead of CD63' by Boumiza et al. Clin Exp Allergy. 2003; 33:849. author reply 852-3. PMID: 12801323.14. Gentinetta T, Pecaric-Petkovic T, Wan D, Falcone FH, Dahinden CA, Pichler WJ, et al. Individual IL-3 priming is crucial for consistent in vitro activation of donor basophils in patients with chronic urticaria. J Allergy Clin Immunol. 2011; 128:1227–1234.e5. PMID: 21855127.15. Marone G, Giugliano R, Lembo G, Ayala F. Human basophil releasability. II. Changes in basophil releasability in patients with atopic dermatitis. J Invest Dermatol. 1986; 87:19–23. PMID: 2425005.
Article16. Sturm EM, Kranzelbinder B, Heinemann A, Groselj-Strele A, Aberer W, Sturm GJ. CD203c-based basophil activation test in allergy diagnosis: characteristics and differences to CD63 upregulation. Cytometry B Clin Cytom. 2010; 78:308–318. PMID: 20533392.
Article17. Wedi B, Novacovic V, Koerner M, Kapp A. Chronic urticaria serum induces histamine release, leukotriene production, and basophil CD63 surface expression--inhibitory effects of anti-inflammatory drugs. J Allergy Clin Immunol. 2000; 105:552–560. PMID: 10719307.
Article18. Chirumbolo S. The use of IL-3 in basophil activation tests is the real pitfall. Cytometry B Clin Cytom. 2011; 80:137–138. author reply 139PMID: 20845369.
Article19. Hausmann OV, Gentinetta T, Fux M, Ducrest S, Pichler WJ, Dahinden CA. Robust expression of CCR3 as a single basophil selection marker in flow cytometry. Allergy. 2011; 66:85–91. PMID: 20608915.
Article20. Ducrest S, Meier F, Tschopp C, Pavlovic R, Dahinden CA. Flowcytometric analysis of basophil counts in human blood and inaccuracy of hematology analyzers. Allergy. 2005; 60:1446–1450. PMID: 16197480.
Article21. Chirumbolo S, Ortolani R, Vella A. CCR3 as a single selection marker compared to CD123/HLADR to isolate basophils in flow cytometry: some comments. Cytometry A. 2011; 79:102–106. PMID: 21265004.
Article22. Ferrer M, Luquin E, Sanchez-Ibarrola A, Moreno C, Sanz ML, Kaplan AP. Secretion of cytokines, histamine and leukotrienes in chronic urticaria. Int Arch Allergy Immunol. 2002; 129:254–260. PMID: 12444324.
Article23. Hennersdorf F, Florian S, Jakob A, Baumgärtner K, Sonneck K, Nordheim A, et al. Identification of CD13, CD107a, and CD164 as novel basophil-activation markers and dissection of two response patterns in time kinetics of IgE-dependent upregulation. Cell Res. 2005; 15:325–335. PMID: 15916720.
Article24. Yasnowsky KM, Dreskin SC, Efaw B, Schoen D, Vedanthan PK, Alam R, et al. Chronic urticaria sera increase basophil CD203c expression. J Allergy Clin Immunol. 2006; 117:1430–1434. PMID: 16751009.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fexofenadine-Induced Urticaria
- Recent applications of basophil activation tests in the diagnosis of drug hypersensitivity
- Increased Level of Basophil CD203c Expression Predicts Severe Chronic Urticaria
- Flow Cytometry-Assisted Basophil Activation Test as a Safe Diagnostic Tool for Aspirin/NSAID Hypersenstivity
- Beef-Induced Anaphylaxis Confirmed by the Basophil Activation Test