Increased Level of Basophil CD203c Expression Predicts Severe Chronic Urticaria
- Affiliations
-
- 1Department of Allergy and Clinical Immunology, Ajou University School of Medicine, Suwon, Korea. hspark@ajou.ac.kr
- KMID: 1796913
- DOI: http://doi.org/10.3346/jkms.2014.29.1.43
Abstract
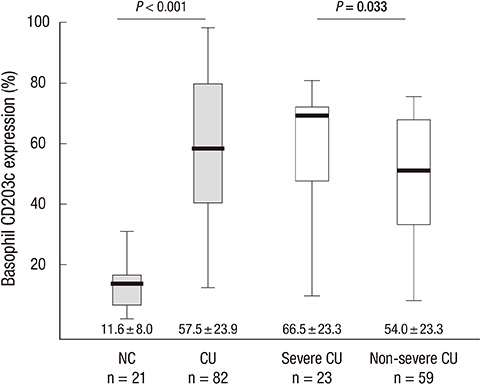
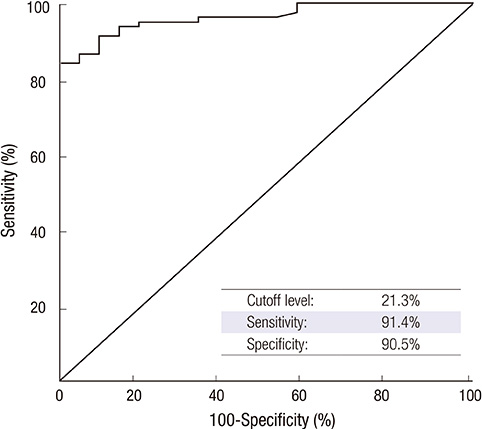
- Increased FcepsilonR1alpha expression with upregulated CD203c expression on peripheral basophils is seen in patients with chronic urticaria (CU). However, there has been no published report on the association between CD203c expression level and clinical disease activity in CU patients. To investigate whether the increase of basophil activation is associated with the disease activity of CU, we measured basophil CD203c expression using a tricolor flow cytometric method in 82 CU patients and 21 normal controls. The relationship between the percentage of CD203c-expressing basophils and clinical parameters was analyzed. The mean basophil CD203c expression was significantly higher in CU patients than in healthy controls (57.5% vs 11.6%, P < 0.001). The basophil CD203c expression in severe CU patients was significantly higher than in non-severe CU (66.5% +/- 23.3% vs 54.0% +/- 23.3%, P = 0.033). Multiple logistic regression analysis indicated that both > or = 72% basophil CD203c expression and urticaria activity score (UAS)> or = 13 were significant predictors of severe CU (P = 0.005 and P = 0.032, respectively). These findings suggest that the quantification of basophil activation with CD203c at baseline may be used as a potential predictor of severe CU requiring another treatment option beyond antihistamines.
MeSH Terms
-
Adult
Autoantibodies/blood
Basophils/*immunology
Female
Flow Cytometry
Humans
Immunoglobulin E/blood/immunology
Male
Phosphoric Diester Hydrolases/biosynthesis/*immunology
Pyrophosphatases/biosynthesis/*immunology
Receptors, IgE/biosynthesis
Urticaria/*immunology
Autoantibodies
Receptors, IgE
Immunoglobulin E
Phosphoric Diester Hydrolases
Pyrophosphatases
Figure
Cited by 4 articles
-
Dimerized, Not Monomeric, Translationally Controlled Tumor Protein Induces Basophil Activation and Mast Cell Degranulation in Chronic Urticaria
Bastsetseg Ulambayar, Heewon Lee, Eun-Mi Yang, Hae-Sim Park, Kyunglim Lee, Young-Min Ye
Immune Netw. 2019;19(3):. doi: 10.4110/in.2019.19.e20.Causal Relationship Between Anti-TPO IgE and Chronic Urticaria by In Vitro and In Vivo Tests
Jorge Sánchez, Andres Sánchez, Ricardo Cardona
Allergy Asthma Immunol Res. 2019;11(1):29-42. doi: 10.4168/aair.2019.11.1.29.Factors Predicting the Response to Cyclosporin Treatment in Patients With Chronic Spontaneous Urticaria: A Systematic Review
Kanokvalai Kulthanan, Chanika Subchookul, Saowalak Hunnangkul, Leena Chularojanamontri, Papapit Tuchinda
Allergy Asthma Immunol Res. 2019;11(5):736-755. doi: 10.4168/aair.2019.11.5.736.KAAACI Work Group report on the management of chronic urticaria
Young-Min Ye, Gwang Cheon Jang, Sun Hee Choi, Jeongmin Lee, Hye-Soo Yoo, Kyung Hee Park, Meeyong Shin, Jihyun Kim, Suh-Young Lee, Jeong-Hee Choi, Youngmin Ahn, Hae-Sim Park, Yoon-Seok Chang, Jae-Won Jeong, Sooyoung Lee,
Allergy Asthma Respir Dis. 2015;3(1):3-14. doi: 10.4168/aard.2015.3.1.3.
Reference
-
1. Zuberbier T, Asero R, Bindslev-Jensen C, Walter Canonica G, Church MK, Giménez-Arnau AM, Grattan CE, Kapp A, Maurer M, Merk HF, et al. EAACI/GA(2)LEN/EDF/WAO guideline: management of urticaria. Allergy. 2009; 64:1427–1443.2. Morgan M, Khan DA. Therapeutic alternatives for chronic urticaria: an evidence-based review, part 1. Ann Allergy Asthma Immunol. 2008; 100:403–411.3. Kaplan AP. Treatment of chronic spontaneous urticaria. Allergy Asthma Immunol Res. 2012; 4:326–331.4. Grattan CE, O'Donnell BF, Francis DM, Niimi N, Barlow RJ, Seed PT, Kobza Black A, Greaves MW. Randomized double-blind study of cyclosporin in chronic 'idiopathic' urticaria. Br J Dermatol. 2000; 143:365–372.5. Nam YH, Kim JH, Jin HJ, Hwang EK, Shin YS, Ye YM, Park HS. Effects of omalizumab treatment in patients with refractory chronic urticaria. Allergy Asthma Immunol Res. 2012; 4:357–361.6. Kessel A, Bishara R, Amital A, Bamberger E, Sabo E, Grushko G, Toubi E. Increased plasma levels of matrix metalloproteinase-9 are associated with the severity of chronic urticaria. Clin Exp Allergy. 2005; 35:221–225.7. Kasperska-Zajac A, Sztylc J, Machura E, Jop G. Plasma IL-6 concentration correlates with clinical disease activity and serum C-reactive protein concentration in chronic urticaria patients. Clin Exp Allergy. 2011; 41:1386–1391.8. Tedeschi A, Lorini M, Suli C, Asero R. Serum interleukin-18 in patients with chronic ordinary urticaria: association with disease activity. Clin Exp Dermatol. 2007; 32:568–570.9. Triwongwaranat D, Kulthanan K, Chularojanamontri L, Pinkaew S. Correlation between plasma D-dimer levels and the severity of patients with chronic urticaria. Asia Pac Allergy. 2013; 3:100–105.10. Zuberbier T, Asero R, Bindslev-Jensen C, Walter Canonica G, Church MK, Giménez-Arnau A, Grattan CE, Kapp A, Merk HF, Rogala B, et al. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009; 64:1417–1426.11. Falcone FH, Knol EF, Gibbs BF. The role of basophils in the pathogenesis of allergic disease. Clin Exp Allergy. 2011; 41:939–947.12. Grattan CE, Dawn G, Gibbs S, Francis DM. Blood basophil numbers in chronic ordinary urticaria and healthy controls: diurnal variation, influence of loratadine and prednisolone and relationship to disease activity. Clin Exp Allergy. 2003; 33:337–341.13. Ito Y, Satoh T, Takayama K, Miyagishi C, Walls AF, Yokozeki H. Basophil recruitment and activation in inflammatory skin diseases. Allergy. 2011; 66:1107–1113.14. Lourenço FD, Azor MH, Santos JC, Prearo E, Maruta CW, Rivitti EA, Duarte AJ, Sato MN. Activated status of basophils in chronic urticaria leads to interleukin-3 hyper-responsiveness and enhancement of histamine release induced by anti-IgE stimulus. Br J Dermatol. 2008; 158:979–986.15. Asero R, Lorini M, Chong SU, Zuberbier T, Tedeschi A. Assessment of histamine-releasing activity of sera from patients with chronic urticaria showing positive autologous skin test on human basophils and mast cells. Clin Exp Allergy. 2004; 34:1111–1114.16. Gentinetta T, Pecaric-Petkovic T, Wan D, Falcone FH, Dahinden CA, Pichler WJ, Hausmann OV. Individual IL-3 priming is crucial for consistent in vitro activation of donor basophils in patients with chronic urticaria. J Allergy Clin Immunol. 2011; 128:1227.e5–1234.e5.17. Chirumbolo S. Basophil activation test in allergy: time for an update. Int Arch Allergy Immunol. 2012; 158:99–114.18. Boumiza R, Monneret G, Forissier MF, Savoye J, Gutowski MC, Powell WS, Bienvenu J. Marked improvement of the basophil activation test by detecting CD203c instead of CD63. Clin Exp Allergy. 2003; 33:259–265.19. Chinuki Y, Kaneko S, Dekio I, Takahashi H, Tokuda R, Nagao M, Fujisawa T, Morita E. CD203c expression-based basophil activation test for diagnosis of wheat-dependent exercise-induced anaphylaxis. J Allergy Clin Immunol. 2012; 129:1404–1406.20. Eckman JA, Hamilton RG, Gober LM, Sterba PM, Saini SS. Basophil phenotypes in chronic idiopathic urticaria in relation to disease activity and autoantibodies. J Invest Dermatol. 2008; 128:1956–1963.21. Yasnowsky KM, Dreskin SC, Efaw B, Schoen D, Vedanthan PK, Alam R, Harbeck RJ. Chronic urticaria sera increase basophil CD203c expression. J Allergy Clin Immunol. 2006; 117:1430–1434.22. Vasagar K, Vonakis BM, Gober LM, Viksman A, Gibbons SP Jr, Saini SS. Evidence of in vivo basophil activation in chronic idiopathic urticaria. Clin Exp Allergy. 2006; 36:770–776.23. Ye YM, Park JW, Kim SH, Choi JH, Hur GY, Lee HY, Lee EH, Park HS. Clinical evaluation of the computerized chronic urticaria-specific quality of life questionnaire in Korean patients with chronic urticaria. Clin Exp Dermatol. 2012; 37:722–728.24. Saini SS, MacGlashan D. How IgE upregulates the allergic response. Curr Opin Immunol. 2002; 14:694–697.25. Schroeder JT, Chichester KL, Bieneman AP. Human basophils secrete IL-3: evidence of autocrine priming for phenotypic and functional responses in allergic disease. J Immunol. 2009; 182:2432–2438.26. Gernez Y, Tirouvanziam R, Yu G, Ghosn EE, Reshamwala N, Nguyen T, Tsai M, Galli SJ, Herzenberg LA, Herzenberg LA, et al. Basophil CD203c levels are increased at baseline and can be used to monitor omalizumab treatment in subjects with nut allergy. Int Arch Allergy Immunol. 2011; 154:318–327.27. Bühring HJ, Streble A, Valent P. The basophil-specific ectoenzyme E-NPP3 (CD203c) as a marker for cell activation and allergy diagnosis. Int Arch Allergy Immunol. 2004; 133:317–329.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Basophil Markers for Identification and Activation in the Indirect Basophil Activation Test by Flow Cytometry for Diagnosis of Autoimmune Urticaria
- Dimerized, Not Monomeric, Translationally Controlled Tumor Protein Induces Basophil Activation and Mast Cell Degranulation in Chronic Urticaria
- Serum Specific IgE to Thyroid Peroxidase Activates Basophils in Aspirin Intolerant Urticaria
- Beef-Induced Anaphylaxis Confirmed by the Basophil Activation Test
- Chlorpheniramine-induced anaphylaxis diagnosed by basophil activation test