J Gastric Cancer.
2013 Sep;13(3):129-135.
Molecular Diagnosis for Personalized Target Therapy in Gastric Cancer
- Affiliations
-
- 1Department of Medical Oncology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. chojy@yuhs.ac
Abstract
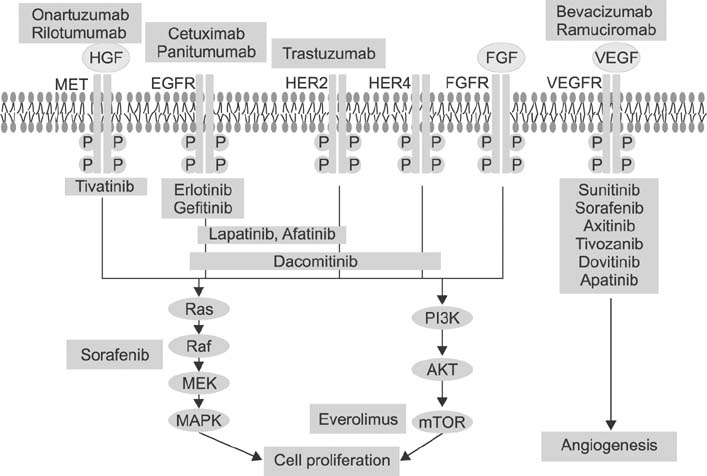
- Gastric cancer is the second leading cause of cancer-related deaths worldwide. In advanced and metastatic gastric cancer, the conventional chemotherapy with limited efficacy shows an overall survival period of about 10 months. Patient specific and effective treatments known as personalized cancer therapy is of significant importance. Advances in high-throughput technologies such as microarray and next generation sequencing for genes, protein expression profiles and oncogenic signaling pathways have reinforced the discovery of treatment targets and personalized treatments. However, there are numerous challenges from cancer target discoveries to practical clinical benefits. Although there is a flood of biomarkers and target agents, only a minority of patients are tested and treated accordingly. Numerous molecular target agents have been under investigation for gastric cancer. Currently, targets for gastric cancer include the epidermal growth factor receptor family, mesenchymal-epithelial transition factor axis, and the phosphatidylinositol 3-kinase-AKT-mammalian target of rapamycin pathways. Deeper insights of molecular characteristics for gastric cancer has enabled the molecular classification of gastric cancer, the diagnosis of gastric cancer, the prediction of prognosis, the recognition of gastric cancer driver genes, and the discovery of potential therapeutic targets. Not only have we deeper insights for the molecular diversity of gastric cancer, but we have also prospected both affirmative potentials and hurdles to molecular diagnostics. New paradigm of transdisciplinary team science, which is composed of innovative explorations and clinical investigations of oncologists, geneticists, pathologists, biologists, and bio-informaticians, is mandatory to recognize personalized target therapy.
MeSH Terms
Figure
Reference
-
1. Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer. 2005; 5:845–856.
Article2. Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, et al. The sequence of the human genome. Science. 2001; 291:1304–1351.
Article3. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
Article4. Shah MA, Ajani JA. Gastric cancer--an enigmatic and heterogeneous disease. JAMA. 2010; 303:1753–1754.
Article5. El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000; 404:398–402.
Article6. Park DW, Lee KJ, Jin SH, Lee JH, Min JS, Park SH, et al. Phenotypic differences of gastric cancer according to the Helicobacter pylori infection in Korean patients. J Gastric Cancer. 2010; 10:168–174.
Article7. Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P, et al. E-cadherin germline mutations in familial gastric cancer. Nature. 1998; 392:402–405.
Article8. Study Group of Millennium Genome Project for Cancer. Study Group, Sakamoto H, Yoshimura K, Saeki N, Katai H, Shimoda T, et al. Genetic variation in PSCA is associated with susceptibility to diffuse-type gastric cancer. Nat Genet. 2008; 40:730–740.
Article9. Tay ST, Leong SH, Yu K, Aggarwal A, Tan SY, Lee CH, et al. A combined comparative genomic hybridization and expression microarray analysis of gastric cancer reveals novel molecular subtypes. Cancer Res. 2003; 63:3309–3316.10. Kim B, Bang S, Lee S, Kim S, Jung Y, Lee C, et al. Expression profiling and subtype-specific expression of stomach cancer. Cancer Res. 2003; 63:8248–8255.11. Chen X, Leung SY, Yuen ST, Chu KM, Ji J, Li R, et al. Variation in gene expression patterns in human gastric cancers. Mol Biol Cell. 2003; 14:3208–3215.
Article12. Lee HS, Cho SB, Lee HE, Kim MA, Kim JH, Park do J, et al. Protein expression profiling and molecular classification of gastric cancer by the tissue array method. Clin Cancer Res. 2007; 13:4154–4163.
Article13. Tan IB, Ivanova T, Lim KH, Ong CW, Deng N, Lee J, et al. Intrinsic subtypes of gastric cancer, based on gene expression pattern, predict survival and respond differently to chemotherapy. Gastroenterology. 2011; 141:476–485.
Article14. Ooi CH, Ivanova T, Wu J, Lee M, Tan IB, Tao J, et al. Oncogenic pathway combinations predict clinical prognosis in gastric cancer. PLoS Genet. 2009; 5:e1000676.
Article15. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastrooesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010; 376:687–697.
Article16. Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011; 29:3968–3976.
Article17. Lieto E, Ferraraccio F, Orditura M, Castellano P, Mura AL, Pinto M, et al. Expression of vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) is an independent prognostic indicator of worse outcome in gastric cancer patients. Ann Surg Oncol. 2008; 15:69–79.
Article18. Tanner M, Hollmén M, Junttila TT, Kapanen AI, Tommola S, Soini Y, et al. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005; 16:273–278.
Article19. Yang J, Luo H, Li Y, Li J, Cai Z, Su X, et al. Intratumoral heterogeneity determines discordant results of diagnostic tests for human epidermal growth factor receptor (HER) 2 in gastric cancer specimens. Cell Biochem Biophys. 2012; 62:221–228.
Article20. Nakajima M, Sawada H, Yamada Y, Watanabe A, Tatsumi M, Yamashita J, et al. The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer. 1999; 85:1894–1902.
Article21. Lee J, Seo JW, Jun HJ, Ki CS, Park SH, Park YS, et al. Impact of MET amplification on gastric cancer: possible roles as a novel prognostic marker and a potential therapeutic target. Oncol Rep. 2011; 25:1517–1524.
Article22. Deng N, Goh LK, Wang H, Das K, Tao J, Tan IB, et al. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut. 2012; 61:673–684.
Article23. Yu G, Wang J, Chen Y, Wang X, Pan J, Li G, et al. Overexpression of phosphorylated mammalian target of rapamycin predicts lymph node metastasis and prognosis of chinese patients with gastric cancer. Clin Cancer Res. 2009; 15:1821–1829.
Article24. Claerhout S, Lim JY, Choi W, Park YY, Kim K, Kim SB, et al. Gene expression signature analysis identifies vorinostat as a candidate therapy for gastric cancer. PLoS One. 2011; 6:e24662.
Article25. Virág L, Szabó C. The therapeutic potential of poly (ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002; 54:375–429.26. Bang YJ. A randomized, open-label, phase III study of lapatinib in combination with weekly paclitaxel versus weekly paclitaxel alone in the second-line treatment of HER2 amplified advanced gastric cancer (AGC) in Asian population: Tytan study. J Clin Oncol. 2012; 30:suppl 34. abstr 11.
Article27. Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, et al. Arbeitsgemeinschaft Internistische Onkologie and EXPAND Investigators. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013; 14:490–499.
Article28. Waddell TS, Chau I, Barbachano Y, Gonzalez de Castro D, Wotherspoon A, Saffery C. A randomized, multicenter trial of epirubicin, oxaliplatin, and capecitabine (EOC) plus panitumumab in advanced esophagogastric cancer (REAL3). J Clin Oncol. 2012; 30:suppl. abstr LBA4000.
Article29. Fuchs CS, Tomasek J, Cho JY, Dumitru F, Passalacqua R, Goswami C, et al. REGARD: a phase III, randomized, double-blinded trial of ramucirumab and best supportive care (BSC) versus placebo and BSC in the treatment of metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma following disease progression on first-line platinum- and/or fluoropyrimidine-containing combination therapy. J Clin Oncol. 2012; 30:suppl 34. abstr LBA5.
Article30. Van Cutsem E, Yeh KH, Bang YJ, Shen L, Ajani JA, Bai YX, et al. Phase III trial of everolimus (EVE) in previously treated patients with advanced gastric cancer (AGC): GRANITE-1. J Clin Oncol. 2012; 30:suppl 4. abstr LBA3.
Article31. Van Cutsem E, de Haas S, Kang YK, Ohtsu A, Tebbutt NC, Ming Xu J, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol. 2012; 30:2119–2127.
Article32. Shah MA, Khanin R, Tang L, Janjigian YY, Klimstra DS, Gerdes H, et al. Molecular classification of gastric cancer: a new paradigm. Clin Cancer Res. 2011; 17:2693–2701.
Article33. Cho JY, Lim JY, Cheong JH, Park YY, Yoon SL, Kim SM, et al. Gene expression signature-based prognostic risk score in gastric cancer. Clin Cancer Res. 2011; 17:1850–1857.
Article34. Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, nodenegative breast cancer. N Engl J Med. 2004; 351:2817–2826.
Article35. Kim YH, Liang H, Liu X, Lee JS, Cho JY, Cheong JH, et al. AMPKα modulation in cancer progression: multilayer integrative analysis of the whole transcriptome in Asian gastric cancer. Cancer Res. 2012; 72:2512–2521.
Article36. Zang ZJ, Cutcutache I, Poon SL, Zhang SL, McPherson JR, Tao J, et al. Exome sequencing of gastric adenocarcinoma identifies recurrent somatic mutations in cell adhesion and chromatin remodeling genes. Nat Genet. 2012; 44:570–574.
Article37. Ueda T, Volinia S, Okumura H, Shimizu M, Taccioli C, Rossi S, et al. Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis. Lancet Oncol. 2010; 11:136–146.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evolution of Gastric Cancer Treatment: From the Golden Age of Surgery to an Era of Precision Medicine
- Personalized Treatment of Head and Neck Cancers and the Role of Head and Neck Surgeons
- Molecularly Targeted Therapy for Lung Cancer : Recent Topics
- Molecular Pathology of Gastric Cancer
- Molecular Biology in Diagnosis and Prognosis of Gastric Cancer