Korean J Physiol Pharmacol.
2016 Jul;20(4):433-440. 10.4196/kjpp.2016.20.4.433.
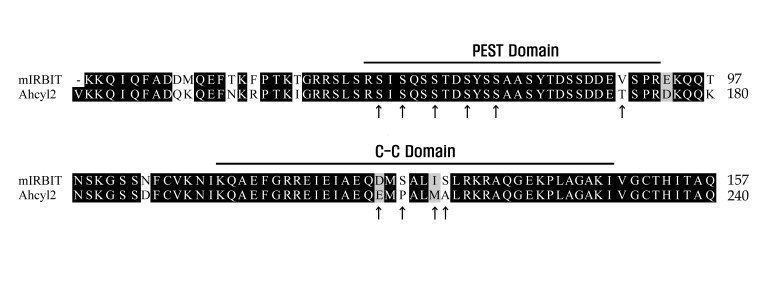
Ahcyl2 upregulates NBCe1-B via multiple serine residues of the PEST domain-mediated association
- Affiliations
-
- 1Department of Laboratory Medicine, Gachon University Gil Hospital, Incheon 21565, Korea.
- 2Department of Physiology, College of Medicine, Gachon University, Incheon 21936, Korea. dkyang@gachon.ac.kr
- KMID: 2371061
- DOI: http://doi.org/10.4196/kjpp.2016.20.4.433
Abstract
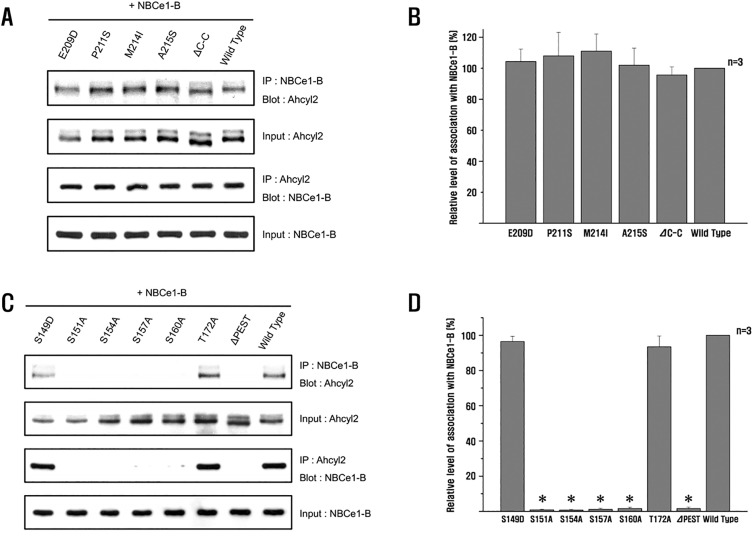
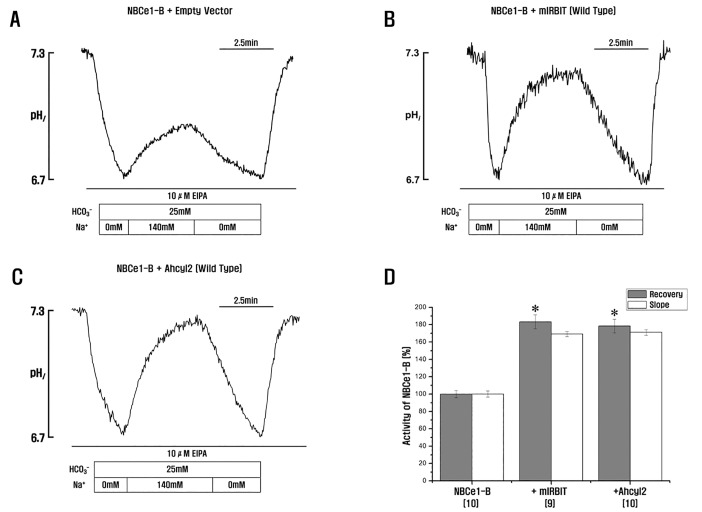
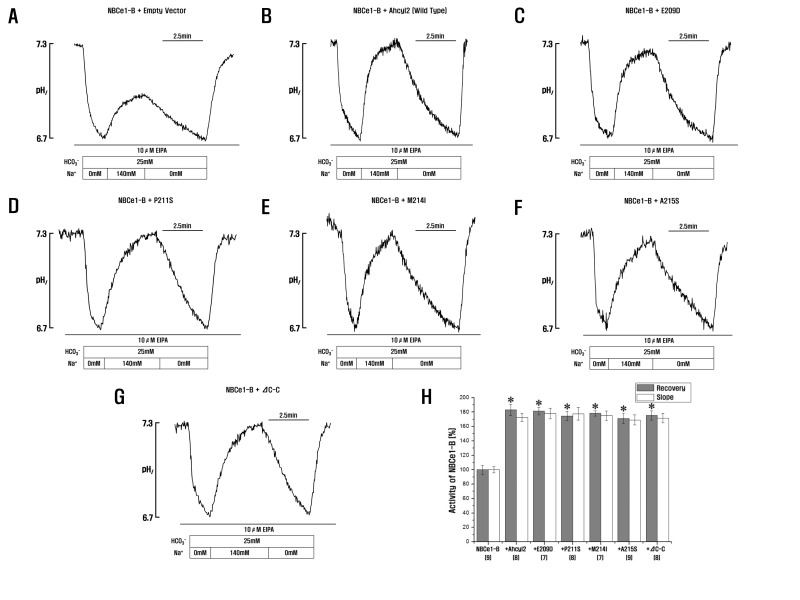
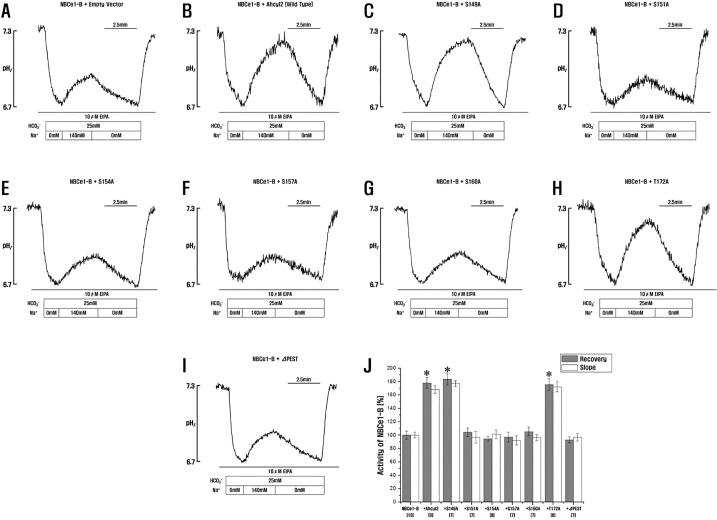
- Inositol-1,4,5-triphosphate [IP3] receptors binding protein released with IP3 (IRBIT) was previously reported as an activator of NBCe1-B. Recent studies have characterized IRBIT homologue S-Adenosylhomocysteine hydrolase-like 2 (AHCYL2). AHCYL2 is highly homologous to IRBIT (88%) and heteromerizes with IRBIT. The two important domains in the N-terminus of AHCYL2 are a PEST domain and a coiled-coil domain which are highly comparable to those in IRBIT. Therefore, in this study, we tried to identify the role of those domains in mouse AHCYL2 (Ahcyl2), and we succeeded in identifying PEST domain of Ahcyl2 as a regulation region for NBCe1-B activity. Site directed mutagenesis and coimmunoprecipitation assay showed that NBCe1-B binds to the N-terminal Ahcyl2-PEST domain, and its binding is determined by the phosphorylation of 4 critical serine residues (Ser151, Ser154, Ser157, and Ser160) in Ahcyl2 PEST domain. Also we revealed that 4 critical serine residues in Ahcyl2 PEST domain are indispensable for the activation of NBCe1-B using measurement of intracellular pH experiment. Thus, these results suggested that the NBCe1-B is interacted with 4 critical serine residues in Ahcyl2 PEST domain, which play an important role in intracellular pH regulation through NBCe1-B.
Keyword
MeSH Terms
Figure
Reference
-
1. Durie PR. The pathophysiology of the pancreatic defect in cystic fibrosis. Acta Paediatr Scand Suppl. 1989; 363:41–44. PMID: 2701923.
Article2. Baron JH. The pancreas. Mt Sinai J Med. 2000; 67:68–75. PMID: 10677785.3. Steward MC, Ishiguro H, Case RM. Mechanisms of bicarbonate secretion in the pancreatic duct. Annu Rev Physiol. 2005; 67:377–409. PMID: 15709963.
Article4. Ishiguro H, Steward MC, Lindsay AR, Case RM. Accumulation of intracellular HCO3– by Na+-HCO3– cotransport in interlobular ducts from guinea-pig pancreas. J Physiol. 1996; 495:169–178. PMID: 8866360.5. Zhao H, Star RA, Muallem S. Membrane localization of H+ and HCO3– transporters in the rat pancreatic duct. J Gen Physiol. 1994; 104:57–85. PMID: 7964596.6. Abuladze N, Lee I, Newman D, Hwang J, Boorer K, Pushkin A, Kurtz I. Molecular cloning, chromosomal localization, tissue distribution, and functional expression of the human pancreatic sodium bicarbonate cotransporter. J Biol Chem. 1998; 273:17689–17695. PMID: 9651366.
Article7. Choi JY, Muallem D, Kiselyov K, Lee MG, Thomas PJ, Muallem S. Aberrant CFTR-dependent HCO3– transport in mutations associated with cystic fibrosis. Nature. 2001; 410:94–97. PMID: 11242048.8. Ko SB, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, Kim JY, Kim KH, Lee MG, Naruse S, Muallem S. A molecular mechanism for aberrant CFTR-dependent HCO3– transport in cystic fibrosis. EMBO J. 2002; 21:5662–5672. PMID: 12411484.9. Lee MG, Choi JY, Luo X, Strickland E, Thomas PJ, Muallem S. Cystic fibrosis transmembrane conductance regulator regulates luminal Cl–/HCO3– exchange in mouse submandibular and pancreatic ducts. J Biol Chem. 1999; 274:14670–14677. PMID: 10329661.10. Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, Muallem S. Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol. 2004; 6:343–350. PMID: 15048129.
Article11. Shcheynikov N, Ko SB, Zeng W, Choi JY, Dorwart MR, Thomas PJ, Muallem S. Regulatory interaction between CFTR and the SLC26 transporters. Novartis Found Symp. 2006; 273:177–186. discussion 186-192, 261-264. PMID: 17120768.
Article12. Wang Y, Soyombo AA, Shcheynikov N, Zeng W, Dorwart M, Marino CR, Thomas PJ, Muallem S. Slc26a6 regulates CFTR activity in vivo to determine pancreatic duct HCO3– secretion: relevance to cystic fibrosis. EMBO J. 2006; 25:5049–5057. PMID: 17053783.13. Dorwart MR, Shcheynikov N, Yang D, Muallem S. The solute carrier 26 family of proteins in epithelial ion transport. Physiology (Bethesda). 2008; 23:104–114. PMID: 18400693.
Article14. Ando H, Mizutani A, Matsu-ura T, Mikoshiba K. IRBIT, a novel inositol 1,4,5-trisphosphate (IP3) receptor-binding protein, is released from the IP3 receptor upon IP3 binding to the receptor. J Biol Chem. 2003; 278:10602–10612. PMID: 12525476.15. Shirakabe K, Priori G, Yamada H, Ando H, Horita S, Fujita T, Fujimoto I, Mizutani A, Seki G, Mikoshiba K. IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein, specifically binds to and activates pancreas-type Na+/HCO3– cotransporter 1 (pNBC1). Proc Natl Acad Sci U S A. 2006; 103:9542–9547. PMID: 16769890.16. Ando H, Mizutani A, Kiefer H, Tsuzurugi D, Michikawa T, Mikoshiba K. IRBIT suppresses IP3 receptor activity by competing with IP3 for the common binding site on the IP3 receptor. Mol Cell. 2006; 22:795–806. PMID: 16793548.17. Devogelaere B, Beullens M, Sammels E, Derua R, Waelkens E, van Lint J, Parys JB, Missiaen L, Bollen M, De Smedt H. Protein phosphatase-1 is a novel regulator of the interaction between IRBIT and the inositol 1,4,5-trisphosphate receptor. Biochem J. 2007; 407:303–311. PMID: 17635105.
Article18. Devogelaere B, Sammels E, De Smedt H. The IRBIT domain adds new functions to the AHCY family. Bioessays. 2008; 30:642–652. PMID: 18536033.
Article19. Hong JH, Yang D, Shcheynikov N, Ohana E, Shin DM, Muallem S. Convergence of IRBIT, phosphatidylinositol (4,5) bisphosphate, and WNK/SPAK kinases in regulation of the Na+-HCO3– cotransporters family. Proc Natl Acad Sci U S A. 2013; 110:4105–4110. PMID: 23431199.20. Yang D, Shcheynikov N, Zeng W, Ohana E, So I, Ando H, Mizutani A, Mikoshiba K, Muallem S. IRBIT coordinates epithelial fluid and HCO3– secretion by stimulating the transporters pNBC1 and CFTR in the murine pancreatic duct. J Clin Invest. 2009; 119:193–202. PMID: 19033647.21. Yamaguchi S, Ishikawa T. AHCYL2 (long-IRBIT) as a potential regulator of the electrogenic Na+-HCO3– cotransporter NBCe1-B. FEBS Lett. 2014; 588:672–677. PMID: 24472682.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Functional identification of protein phosphatase 1-binding consensus residues in NBCe1-B
- NBCe1 Regulates Odontogenic Differentiation of Human Dental Pulp Stem Cells via NF-κB
- Effect of serine protease inhibitor on follicular development in the rat ovary
- Overexpressed Mitochondrial Thioredoxin Protects PC12 Cells from Hydrogen Peroxide and Serum-deprivation
- Binding of the Streptococcus gordonii Surface Glycoprotein Hsa to alpha(2-3) Linked Sialic Acid Residues on Fibronectin