J Bacteriol Virol.
2014 Dec;44(4):317-325. 10.4167/jbv.2014.44.4.317.
Binding of the Streptococcus gordonii Surface Glycoprotein Hsa to alpha(2-3) Linked Sialic Acid Residues on Fibronectin
- Affiliations
-
- 1Radiation Biotechnology Research Division, Advanced Radiation Technology Institute, Korea Atomic Energy Research Institute, JeongEup Si, Korea. hoseongseo@kaeri.re.kr
- KMID: 1737320
- DOI: http://doi.org/10.4167/jbv.2014.44.4.317
Abstract
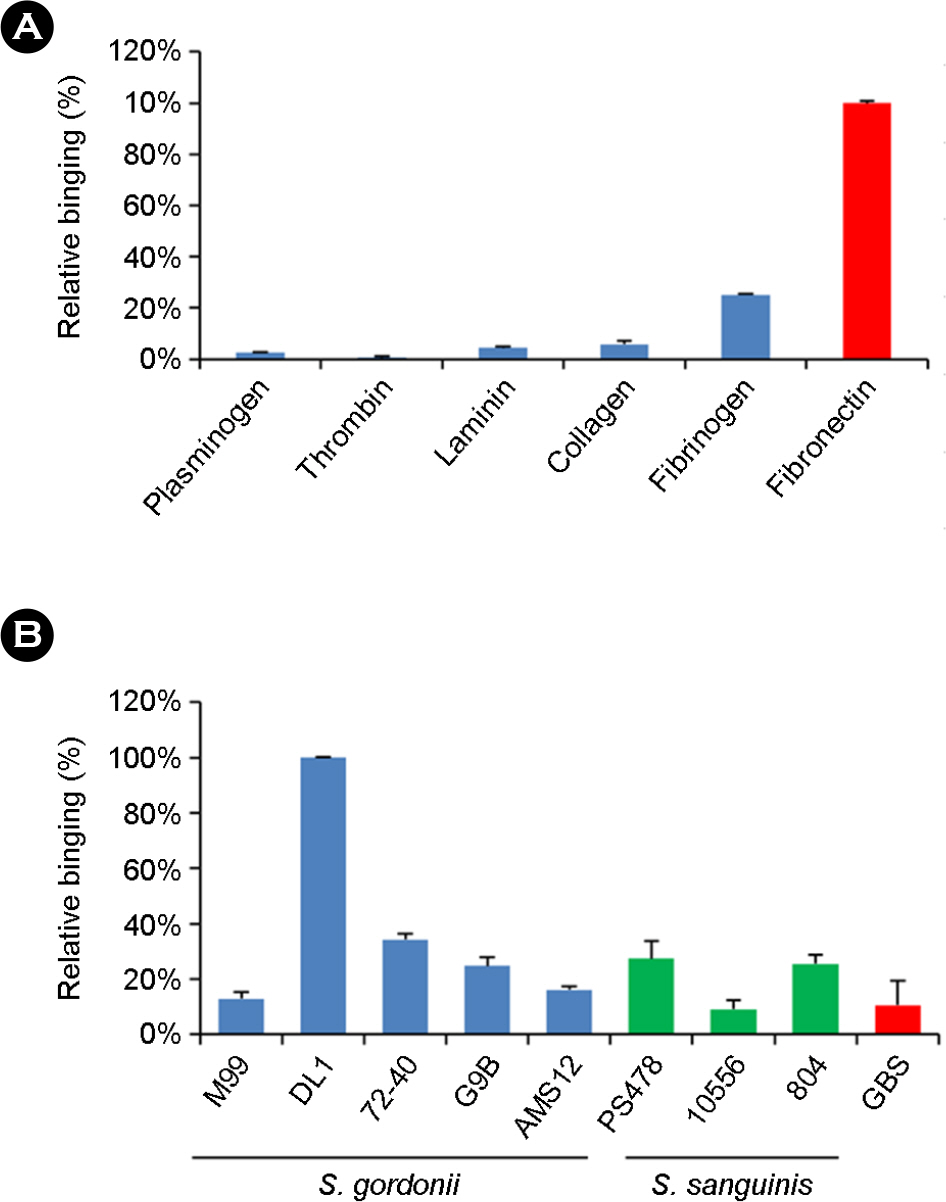
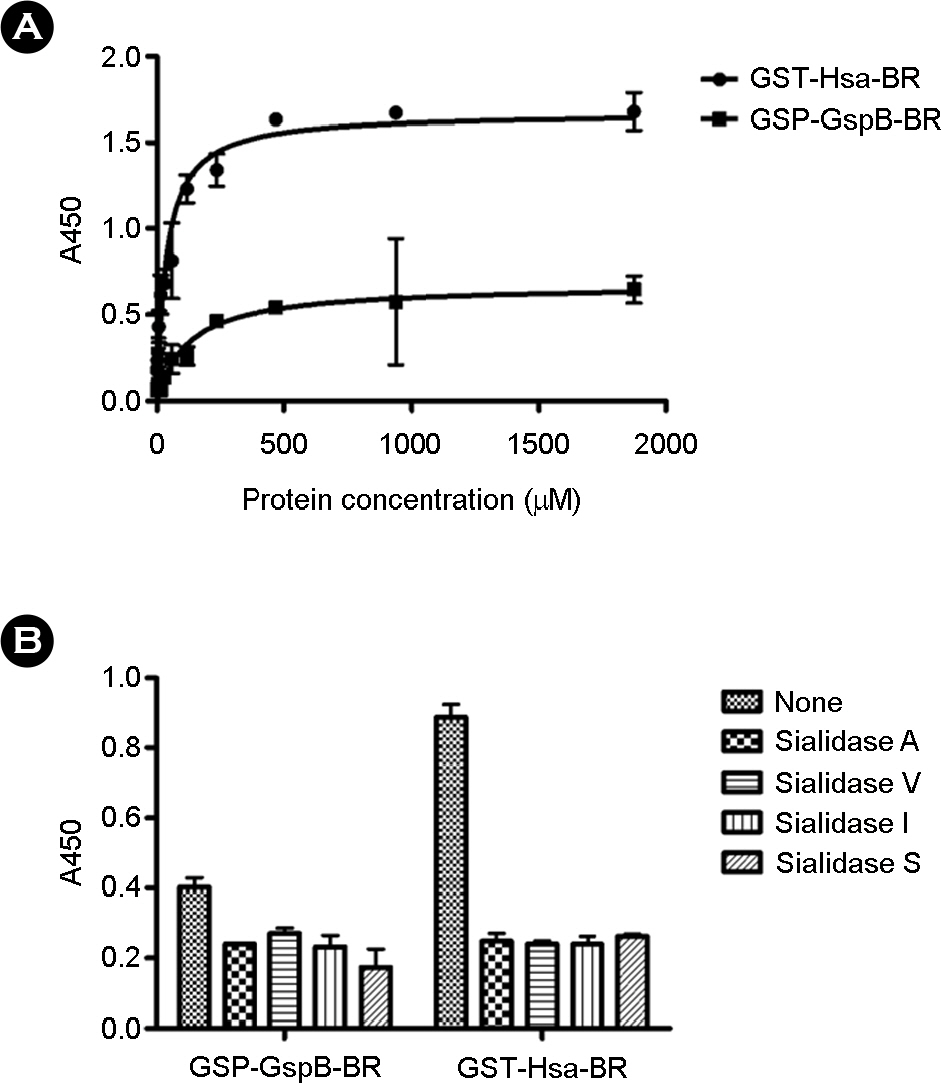
- The binding of microorganisms to platelets is a critical step in the development of infective endocarditis. In Streptococcus gordonii, this binding is mediated in part by serine-rich repeat proteins, which interact directly with sialic acid residues located on GPIIb receptors in the platelet membrane. In this study, we found that S. gordonii DL1 strain binds to platelets through bridging between sialic acid residue of fibronectin and serine-rich repeat protein (Hsa). Pretreatment of fibronectin with sialidases specific for alpha(2-3)-linked sialic acids was shown to significantly inhibit binding of the DL1 strain and the binding region(BR) of Hsa protein. Similarly, pre-incubation of bacteria or BR of Hsa with alpha(2-3)-sialyl-N-acetyllactosamine blocked fibronectin binding in the DL1 strain, but not the M99 strain. Together, these data show that the alpha(2-3)-sialic acid residues of fibronectin play an important role in the binding of S. gordonii DL1 to fibronectin through interactions with the Hsa receptor. This interaction is thought to play an important role in the development of pathogenic endocarditis, and may represent an important therapeutic target for the treatment of infective endocarditis.
MeSH Terms
Figure
Reference
-
1). Werdan K, Dietz S, Löffler B, Niemann S, Bushnaq H, Silber RE, et al. Mechanisms of infective endocarditis: pathogen-host interaction and risk states. Nat Rev Cardiol. 2014; 11:35–50.
Article2). Thuny F, Grisoli D, Cautela J, Riberi A, Raoult D, Habib G. Infective endocarditis: prevention, diagnosis, and management. Can J Cardiol. 2014; 30:1046–57.
Article3). Ferro JM, Fonseca AC. Infective endocarditis. Handb Clin Neurol. 2014; 119:75–91.
Article4). Keane C, Petersen HJ, Tilley DO, Haworth J, Cox D, Jenkinson HF, et al. Multiple sites on Streptococcus gordonii surface protein PadA bind to platelet GPIIbIIIa. Thromb Haemost. 2013; 110:1278–87.5). Seo HS, Xiong YQ, Mitchell J, Seepersaud R, Bayer AS, Sullam PM. Bacteriophage lysin mediates the binding of Streptococcus mitis to human platelets through interaction with fibrinogen. PLoS Pathog. 2010; 6:e1001047.6). Yamaguchi T, Soutome S, Oho T. Identification and characterization of a fibronectin-binding protein from Granulicatella adiacens. Mol Oral Microbiol. 2011; 26:353–64.
Article7). Heilmann C, Niemann S, Sinha B, Herrmann M, Kehrel BE, Peters G. Staphylococcus aureus fibronectin-binding protein (FnBP)-mediated adherence to platelets, and aggregation of platelets induced by FnBPA but not by FnBPB. J Infect Dis. 2004; 190:321–9.8). Sommer P, Gleyzal C, Guerret S, Etienne J, Grimaud JA. Induction of a putative laminin-binding protein of Streptococcus gordonii in human infective endocarditis. Infect Immun. 1992; 60:360–5.9). Takamatsu D, Bensing BA, Prakobphol A, Fisher SJ, Sullam PM. Binding of the streptococcal surface glycoproteins GspB and Hsa to human salivary proteins. Infect Immun. 2006; 74:1933–40.
Article10). Lim HH, Yoo YS, Kim KW, Kook JK. Frequency of Species and Biotypes of Mutans Streptococci Isolated from Dental Plaque in the Adolescents and Adults. J Bacteriol Virol. 2005; 35:197–201.11). Jung CJ, Yeh CY, Shun CT, Hsu RB, Cheng HW, Lin CS, et al. Platelets enhance biofilm formation and resistance of endocarditis-inducing streptococci on the injured heart valve. J Infect Dis. 2012; 205:1066–75.
Article12). Mitchell J, Sullam PM. Streptococcus mitis phagencoded adhesins mediate attachment to α2-8-linked sialic acid residues on platelet membrane gangliosides. Infect Immun. 2009; 77:3485–90.13). Yajima A, Urano-Tashiro Y, Shimazu K, Takashima E, Takahashi Y, Konishi K. Hsa, an adhesin of Streptococcus gordonii DL1, binds to alpha2-3-linked sialic acid on glycophorin A of the erythrocyte membrane. Microbiol Immunol. 2008; 52:69–77.14). George NP, Wei Q, Shin PK, Konstantopoulos K, Ross JM. Staphylococcus aureus adhesion via Spa, ClfA, and SdrCDE to immobilized platelets demonstrates shear-dependent behavior. Arterioscler Thromb Vasc Biol. 2006; 26:2394–400.15). Fitzgerald JR, Loughman A, Keane F, Brennan M, Knobel M, Higgins J, et al. Fibronectin-binding proteins of Staphylococcus aureus mediate activation of human platelets via fibrinogen and fibronectin bridges to integrin GPIIb/IIIa and IgG binding to the FcgammaRIIa receptor. Mol Microbiol. 2006; 59:212–30.16). Fitzgerald JR, Foster TJ, Cox D. The interaction of bacterial pathogens with platelets. Nat Rev Microbiol. 2006; 4:445–57.
Article17). Shin SG, Koh SH, Lim JH. The in vivo and in vitro Roles of Epithelial Pattern Recognition Receptors in Pneumococcal Infections. J Bacteriol Virol. 2014; 44:121–32.18). Lee SY, Lee JS, Choe SJ. Bactericidal Activity of Thrombin - induced Platelet Microbicidal Protein Against Streptococcus rattus BHT. J Bacteriol Virol. 2001; 31:317–24.19). Pyburn TM, Bensing BA, Xiong YQ, Melancon BJ, Tomasiak TM, Ward NJ, et al. A structural model for binding of the serine-rich repeat adhesin GspB to host carbohydrate receptors. PLoS Pathog. 2011; 7:e1002112.
Article20). Takamatsu D, Bensing BA, Cheng H, Jarvis GA, Siboo IR, López JA, et al. Binding of the Streptococcus gordonii surface glycoproteins GspB and Hsa to specific carbohydrate structures on platelet membrane glycoprotein Ibalpha. Mol Microbiol. 2005; 58:380–92.21). Xiong YQ, Bensing BA, Bayer AS, Chambers HF, Sullam PM. Role of the serine-rich surface glycoprotein GspB of Streptococcus gordonii in the pathogenesis of infective endocarditis. Microb Pathog. 2008; 45:297–301.22). Kerrigan SW, Jakubovics NS, Keane C, Maguire P, Wynne K, Jenkinson HF, et al. Role of Streptococcus gordonii surface proteins SspA/SspB and Hsa in platelet function. Infect Immun. 2007; 75:5740–7.23). Sullam PM, Frank U, Yeaman MR, Täuber MG, Bayer AS, Chambers HF. Effect of thrombocytopenia on the early course of streptococcal endocarditis. J Infect Dis. 1993; 168:910–4.
Article24). Seo HS, Minasov G, Seepersaud R, Doran KS, Dubrovska I, Shuvalova L, et al. Characterization of fibrinogen binding by glycoproteins Srr1 and Srr2 of Streptococcus agalactiae. J Biol Chem. 2013; 288:35982–96.25). Jakubovics NS, Brittan JL, Dutton LC, Jenkinson HF. Multiple adhesin proteins on the cell surface of Streptococcus gordonii are involved in adhesion to human fibronectin. Microbiology. 2009; 155:3572–80.26). Yajima A, Takahashi Y, Konishi K. Identification of platelet receptors for the Streptococcus gordonii DL1 sialic acid-binding adhesin. Microbiol Immunol. 2005; 49:795–800.27). Bensing BA, López JA, Sullam PM. The Streptococcus gordonii surface proteins GspB and Hsa mediate binding to sialylated carbohydrate epitopes on the platelet membrane glycoprotein Ibalpha. Infect Immun. 2004; 72:6528–37.28). Halper J, Kjaer M. Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv Exp Med Biol. 2014; 802:31–47.
Article29). Holmes AR, McNab R, Millsap KW, Rohde M, Hammerschmidt S, Mawdsley JL, et al. The pavA gene of Streptococcus pneumoniae encodes a fibronectin-binding protein that is essential for virulence. Mol Microbiol. 2001; 41:1395–408.30). McNab R, Holmes AR, Clarke JM, Tannock GW, Jenkinson HF. Cell surface polypeptide CshA mediates binding of Streptococcus gordonii to other oral bacteria and to immobilized fibronectin. Infect Immun. 1996; 64:4204–10.31). Katerov V, Andreev A, Schalén C, Totolian AA. Protein F, a fibronectin-binding protein of Streptococcus pyogenes, also binds human fibrinogen: isolation of the protein and mapping of the binding region. Microbiology. 1998; 144(Pt 1):119–26.32). Geoghegan JA, Monk IR, O'Gara JP, Foster TJ. Subdomains N2N3 of fibronectin binding protein A mediate Staphylococcus aureus biofilm formation and adherence to fibrinogen using distinct mechanisms. J Bacteriol. 2013; 195:2675–83.33). Bingham RJ, Rñdiño-Pinera E, Meenan NA, Schwarz-Linek U, Turkenburg JP, Höök M, et al. Crystal structures of fibronectin-binding sites from Staphylococcus aureus FnBPA in complex with fibronectin domains. Proc Natl Acad Sci U S A. 2008; 105:12254–8.34). Peacock SJ, Day NP, Thomas MG, Berendt AR, Foster TJ. Clinical isolates of Staphylococcus aureus exhibit diversity in fnb genes and adhesion to human fibronectin. J Infect. 2000; 41:23–31.35). Heying R, van de Gevel J, Que YA, Moreillon P, Beekhuizen H. Fibronectin-binding proteins and clumping factor A in Staphylococcus aureus experimental endocarditis: FnBPA is sufficient to activate human endothelial cells. Thromb Haemost. 2007; 97:617–26.36). Bensing BA, Takamatsu D, Sullam PM. Determinants of the streptococcal surface glycoprotein GspB that facilitate export by the accessory Sec system. Mol Microbiol. 2005; 58:1468–81.
Article37). Bensing BA, Gibson BW, Sullam PM. The Streptococcus gordonii platelet binding protein GspB undergoes glycosylation independently of export. J Bacteriol. 2004; 186:638–45.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Characterization of Congo red binding in Streptococcus gordonii
- Effect of Subinhibitory Concentrations of Antibiotics on Cell Surface Properties of Streptococcus gordonii and Staphylococcus aureus
- Tissue distribution of sialic acid-linked influenza virus receptors in beagle dogs
- Expression of Sialic Acids according to the Differentiation of Cultured Human Nasal Epithelial Cells
- New Insights into the Mechanism of the Down-Regulation of Mast Cells in the Treatment of Interstitial Cystitis: Possible Role of Siglecs