Korean J Physiol Pharmacol.
2016 Jul;20(4):425-432. 10.4196/kjpp.2016.20.4.425.
Facilitation of AMPA receptor-mediated steady-state current by extrasynaptic NMDA receptors in supraoptic magnocellular neurosecretory cells
- Affiliations
-
- 1Department of Physiology, Brain Research Institute, School of Medicine, Chungnam National University, Daejeon 35015, Korea. jinbong@cnu.ac.kr seohwy@cnu.ac.kr
- 2Department of Anesthesiology & Pain Medicine, Brain Research Institute, School of Medicine, Chungnam National University, Daejeon 35015, Korea. jinbong@cnu.ac.kr seohwy @cnu.ac.kr
- KMID: 2371060
- DOI: http://doi.org/10.4196/kjpp.2016.20.4.425
Abstract
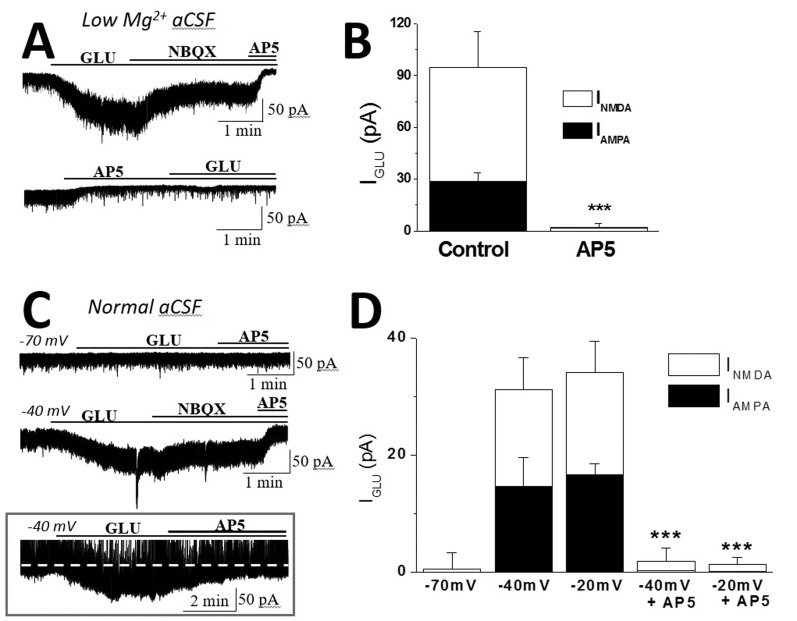
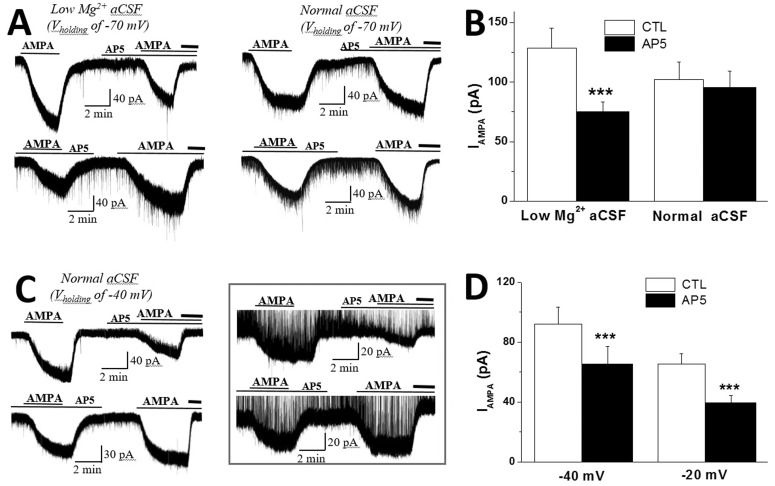
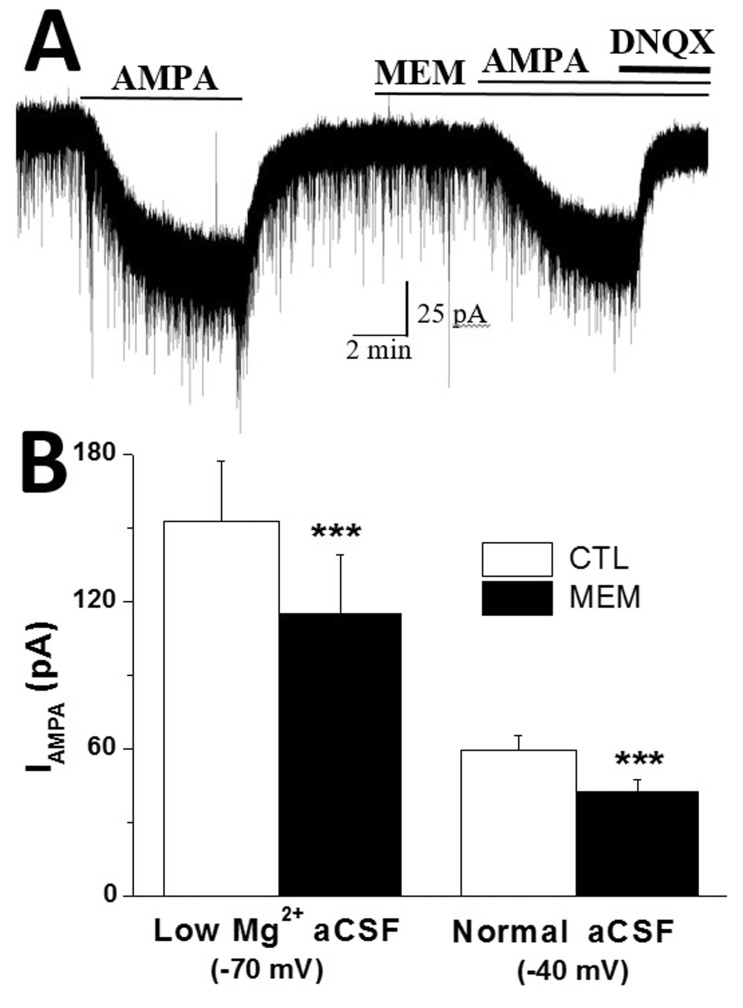
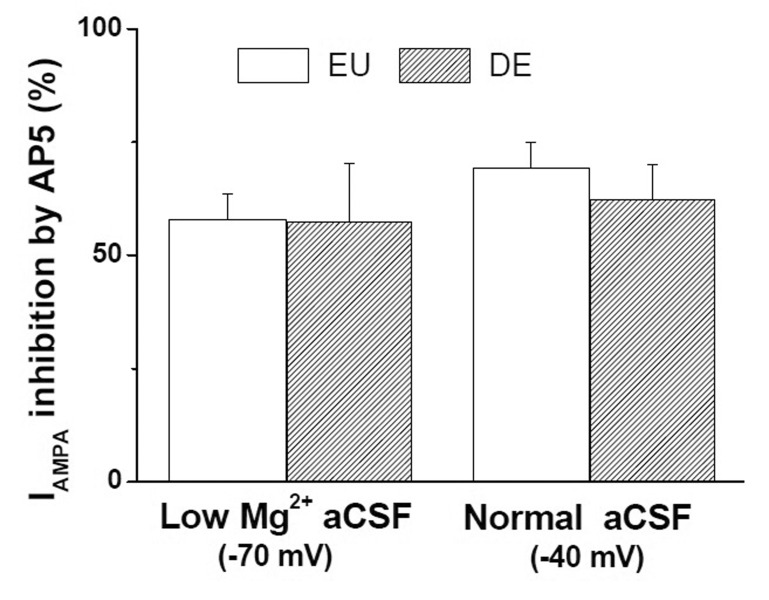
- In addition to classical synaptic transmission, information is transmitted between cells via the activation of extrasynaptic receptors that generate persistent tonic current in the brain. While growing evidence supports the presence of tonic NMDA current (INMDA) generated by extrasynaptic NMDA receptors (eNMDARs), the functional significance of tonic I(NMDA) in various brain regions remains poorly understood. Here, we demonstrate that activation of eNMDARs that generate I(NMDA) facilitates the α-amino-3-hydroxy-5-methylisoxazole-4-proprionate receptor (AMPAR)-mediated steady-state current in supraoptic nucleus (SON) magnocellular neurosecretory cells (MNCs). In low-Mg2+ artificial cerebrospinal fluid (aCSF), glutamate induced an inward shift in I(holding) (I(GLU)) at a holding potential (V(holding)) of -70 mV which was partly blocked by an AMPAR antagonist, NBQX. NBQX-sensitive I(GLU) was observed even in normal aCSF at V(holding) of -40 mV or -20 mV. I(GLU) was completely abolished by pretreatment with an NMDAR blocker, AP5, under all tested conditions. AMPA induced a reproducible inward shift in I(holding) (I(AMPA)) in SON MNCs. Pretreatment with AP5 attenuated I(AMPA) amplitudes to ~60% of the control levels in low-Mg2+ aCSF, but not in normal aCSF at V(holding) of -70 mV. I(AMPA) attenuation by AP5 was also prominent in normal aCSF at depolarized holding potentials. Memantine, an eNMDAR blocker, mimicked the AP5-induced I(AMPA) attenuation in SON MNCs. Finally, chronic dehydration did not affect I(AMPA) attenuation by AP5 in the neurons. These results suggest that tonic I(NMDA), mediated by eNMDAR, facilitates AMPAR function, changing the postsynaptic response to its agonists in normal and osmotically challenged SON MNCs.
Keyword
MeSH Terms
-
alpha-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid*
Brain
Cerebrospinal Fluid
Dehydration
Glutamic Acid
Memantine
N-Methylaspartate*
Neurons
Receptors, AMPA
Receptors, N-Methyl-D-Aspartate*
Supraoptic Nucleus
Synaptic Transmission
Glutamic Acid
Memantine
N-Methylaspartate
Receptors, AMPA
Receptors, N-Methyl-D-Aspartate
alpha-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid
Figure
Reference
-
1. Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005; 6:215–229. PMID: 15738957.
Article2. Makani S, Zagha E. Out of the cleft: the source and target of extrasynaptic glutamate in the CA1 region of the hippocampus. J Physiol. 2007; 582:479–480. PMID: 17556385.
Article3. Pandit S, Jo JY, Lee SU, Lee YJ, Lee SY, Ryu PD, Lee JU, Kim HW, Jeon BH, Park JB. Enhanced astroglial GABA uptake attenuates tonic GABAA inhibition of the presympathetic hypothalamic paraventricular nucleus neurons in heart failure. J Neurophysiol. 2015; 114:914–926. PMID: 26063771.
Article4. Potapenko ES, Biancardi VC, Zhou Y, Stern JE. Altered astrocyte glutamate transporter regulation of hypothalamic neurosecretory neurons in heart failure rats. Am J Physiol Regul Integr Comp Physiol. 2012; 303:R291–R300. PMID: 22696576.
Article5. Dalby NO, Mody I. Activation of NMDA receptors in rat dentate gyrus granule cells by spontaneous and evoked transmitter release. J Neurophysiol. 2003; 90:786–797. PMID: 12904493.6. Fleming TM, Scott V, Naskar K, Joe N, Brown CH, Stern JE. State-dependent changes in astrocyte regulation of extrasynaptic NMDA receptor signalling in neurosecretory neurons. J Physiol. 2011; 589:3929–3941. PMID: 21690192.
Article7. Le Meur K, Galante M, Angulo MC, Audinat E. Tonic activation of NMDA receptors by ambient glutamate of non-synaptic origin in the rat hippocampus. J Physiol. 2007; 580:373–383. PMID: 17185337.8. Sah P, Hestrin S, Nicoll RA. Tonic activation of NMDA receptors by ambient glutamate enhances excitability of neurons. Science. 1989; 246:815–818. PMID: 2573153.
Article9. Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002; 5:405–414. PMID: 11953750.
Article10. Ivanov A, Pellegrino C, Rama S, Dumalska I, Salyha Y, Ben-Ari Y, Medina I. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. J Physiol. 2006; 572:789–798. PMID: 16513670.
Article11. Okamoto S, Pouladi MA, Talantova M, Yao D, Xia P, Ehrnhoefer DE, Zaidi R, Clemente A, Kaul M, Graham RK, Zhang D, Vincent Chen HS, Tong G, Hayden MR, Lipton SA. Balance between synaptic versus extrasynaptic NMDA receptor activity influences inclusions and neurotoxicity of mutant huntingtin. Nat Med. 2009; 15:1407–1413. PMID: 19915593.
Article12. Pougnet JT, Toulme E, Martinez A, Choquet D, Hosy E, Boué-Grabot E. ATP P2X receptors downregulate AMPA receptor trafficking and postsynaptic efficacy in hippocampal neurons. Neuron. 2014; 83:417–430. PMID: 25033184.
Article13. Ferreira-Neto HC, Antunes VR, Stern JE. ATP stimulates rat hypothalamic sympathetic neurons by enhancing AMPA receptor-mediated currents. J Neurophysiol. 2015; 114:159–169. PMID: 25904713.
Article14. Gordon GR, Baimoukhametova DV, Hewitt SA, Rajapaksha WR, Fisher TE, Bains JS. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat Neurosci. 2005; 8:1078–1086. PMID: 15995701.
Article15. Silverman AJ, Zimmerman EA. Magnocellular neurosecretory system. Annu Rev Neurosci. 1983; 6:357–380. PMID: 6301350.
Article16. van den Pol AN, Wuarin JP, Dudek FE. Glutamate, the dominant excitatory transmitter in neuroendocrine regulation. Science. 1990; 250:1276–1278. PMID: 1978759.
Article17. Park JB, Skalska S, Stern JE. Characterization of a novel tonic gamma-aminobutyric acidA receptor-mediated inhibition in magnocellular neurosecretory neurons and its modulation by glia. Endocrinology. 2006; 147:3746–3760. PMID: 16675519.18. Jeong JA, Kim EJ, Jo JY, Song JG, Lee KS, Kim HW, Lee SD, Jeon BH, Lee JU, Park JB. Major role of GABA(A)-receptor mediated tonic inhibition in propofol suppression of supraoptic magnocellular neurons. Neurosci Lett. 2011; 494:119–123. PMID: 21376783.
Article19. Jo JY, Jeong JA, Pandit S, Stern JE, Lee SK, Ryu PD, Lee SY, Han SK, Cho CH, Kim HW, Jeon BH, Park JB. Neurosteroid modulation of benzodiazepine-sensitive GABAA tonic inhibition in supraoptic magnocellular neurons. Am J Physiol Regul Integr Comp Physiol. 2011; 300:R1578–R1587. PMID: 21451144.
Article20. Park JB, Skalska S, Son S, Stern JE. Dual GABAA receptor-mediated inhibition in rat presympathetic paraventricular nucleus neurons. J Physiol. 2007; 582:539–551. PMID: 17495040.21. Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat Rev Drug Discov. 2006; 5:160–170. PMID: 16424917.
Article22. Papadia S, Soriano FX, Léveillé F, Martel MA, Dakin KA, Hansen HH, Kaindl A, Sifringer M, Fowler J, Stefovska V, McKenzie G, Craigon M, Corriveau R, Ghazal P, Horsburgh K, Yankner BA, Wyllie DJ, Ikonomidou C, Hardingham GE. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci. 2008; 11:476–487. PMID: 18344994.
Article23. Di S, Tasker JG. Dehydration-induced synaptic plasticity in magnocellular neurons of the hypothalamic supraoptic nucleus. Endocrinology. 2004; 145:5141–5149. PMID: 15297447.
Article24. Papouin T, Oliet SH. Organization, control and function of extrasynaptic NMDA receptors. Philos Trans R Soc Lond B Biol Sci. 2014; 369:20130601. PMID: 25225095.
Article25. Papouin T, Ladépêche L, Ruel J, Sacchi S, Labasque M, Hanini M, Groc L, Pollegioni L, Mothet JP, Oliet SH. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell. 2012; 150:633–646. PMID: 22863013.
Article26. Borgdorff AJ, Choquet D. Regulation of AMPA receptor lateral movements. Nature. 2002; 417:649–653. PMID: 12050666.
Article27. Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003; 40:361–379. PMID: 14556714.
Article28. Choquet D, Triller A. The role of receptor diffusion in the organization of the postsynaptic membrane. Nat Rev Neurosci. 2003; 4:251–265. PMID: 12671642.
Article29. Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002; 25:103–126. PMID: 12052905.
Article30. Derkach V, Barria A, Soderling TR. Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc Natl Acad Sci U S A. 1999; 96:3269–3274. PMID: 10077673.31. Fukunaga K, Stoppini L, Miyamoto E, Muller D. Long-term potentiation is associated with an increased activity of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1993; 268:7863–7867. PMID: 8385124.32. Theodosis DT, Poulain DA. Activity-dependent neuronalglial and synaptic plasticity in the adult mammalian hypothalamus. Neuroscience. 1993; 57:501–535. PMID: 8309521.
Article33. Oliet SH, Piet R, Poulain DA. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science. 2001; 292:923–926. PMID: 11340204.
Article34. Angulo MC, Kozlov AS, Charpak S, Audinat E. Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J Neurosci. 2004; 24:6920–6927. PMID: 15295027.
Article35. Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004; 43:729–743. PMID: 15339653.
Article36. Fiacco TA, McCarthy KD. Intracellular astrocyte calcium waves in situ increase the frequency of spontaneous AMPA receptor currents in CA1 pyramidal neurons. J Neurosci. 2004; 24:722–732. PMID: 14736858.
Article37. Liu QS, Xu Q, Arcuino G, Kang J, Nedergaard M. Astrocyte-mediated activation of neuronal kainate receptors. Proc Natl Acad Sci U S A. 2004; 101:3172–3177. PMID: 14766987.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Electrophysiological Characterization of AMPA and NMDA Receptors in Rat Dorsal Striatum
- Inhibitory and Excitatory Postsynaptic Currents of Medial Vestibular Nucleus Neurons of Rats
- A Possible Role of Kainate Receptors in C2C12 Skeletal Myogenic Cells
- A Role of Glutamate and GABA Receptors in the Generation of Formalin-induced Impulse Firing of Spinal Dorsal Horn Neurons in the Rat
- AMPA, not NMDA, activates RhoA GTPases and subsequetly phosphorylates moesin